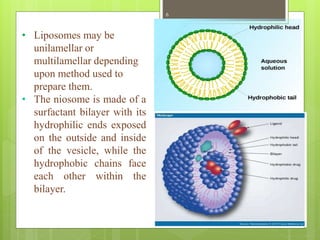
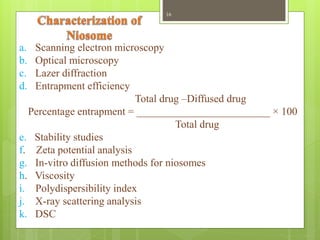
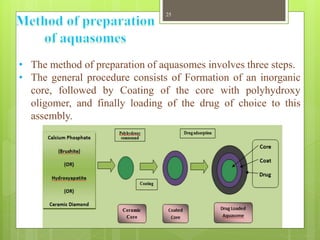
This document provides an overview of Niosomes and Aquasomes as novel drug delivery systems. Niosomes are vesicle systems composed of non-ionic surfactants that can encapsulate medications. They are prepared using methods like ether injection, thin film hydration, sonication, and offer advantages over liposomes. Aquasomes are three-layered, self-assembled nanoparticle structures with a solid inorganic core coated with oligomers and bioactive molecules. Both systems can be characterized and show applications in targeted drug delivery, diagnostics, and improving drug stability and efficacy.