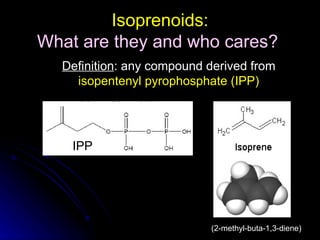
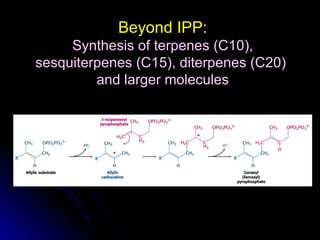
This document discusses isoprenoids, which are compounds derived from isopentenyl pyrophosphate (IPP). It summarizes that isoprenoids play important roles in animals, plants, and modern life. It also describes a method developed by Keller and Thompson to efficiently synthesize isoprenoid pyrophosphate intermediates using bis(triethylammonium) phosphate and trichloroacetonitrile, followed by silica chromatography. This procedure allows rapid preparation of isoprenoid pyrophosphates that are widely used in studies of isoprenoid metabolism.















![TLC of products of [ 3 H]Farnesol-bisTEAP reaction](https://image.slidesharecdn.com/LinkedInslideshow-123181481234-phpapp01/85/Isoprenoids-19-320.jpg)