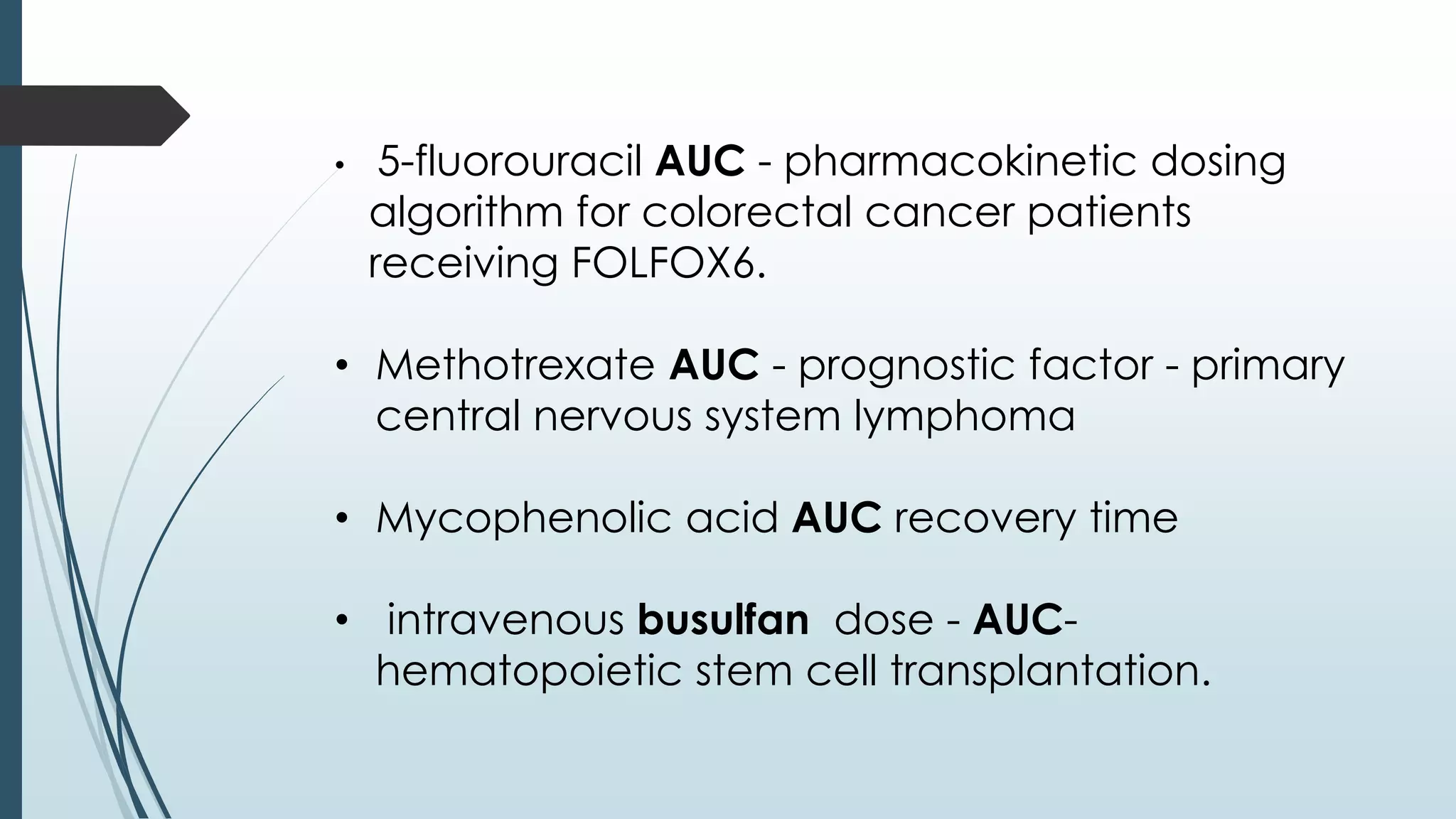
The document discusses area under the curve (AUC) as it relates to bioavailability and pharmacokinetics. It defines AUC as the definite integral of the plasma drug concentration-time curve, which provides a measure of total drug exposure. Various methods for calculating AUC are described, including trapezoidal rule, which divides the curve into trapezoids and sums their areas. Factors affecting bioavailability and AUC include drug properties, formulation, and patient factors. Clinical applications of AUC include toxicity assessment, bioequivalence studies, and pharmacokinetic dosing.





































![AUC1/W1 = AUC2/W2
AUC2= 192 ug.hr/ml
W1= 790mg
W2= 2750mh
AUC1= [(192)(790) / 2750 ] = 55.15ug.hr/ml
Units Y axis ug/ml nd X axis is Hours so area
is ug.Hr/ml](https://image.slidesharecdn.com/areaunderthecurve123-150501002026-conversion-gate02/75/Area-under-the-curve-Dr-ASHWIN-R-38-2048.jpg)