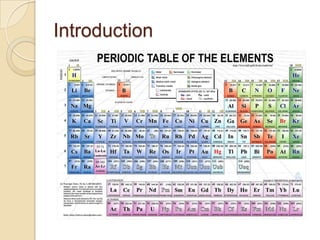
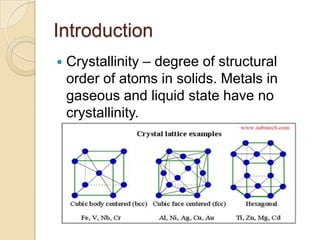
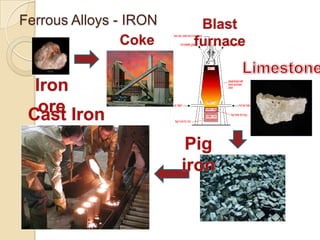
Metals can be classified as metallic, metalloids, or nonmetallic. Metallic properties include hardness, brittleness, malleability, ductility, elasticity, toughness, density, fusibility, conduction, contraction, expansion, and strength. Alloys are formed by melting two metals together. The most important industrial alloys are based on iron, lead, copper, zinc, tin, nickel, magnesium, and aluminum. Metals can be treated through hot/cold working and heat treatment to modify properties without changing shape. Common commercial metals include steel, iron, titanium alloys, superalloys, aluminum alloys, and intermetallic compounds.