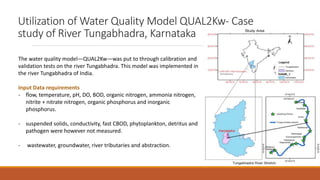
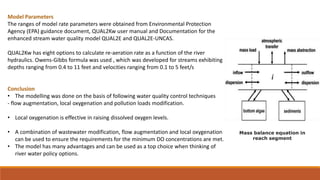
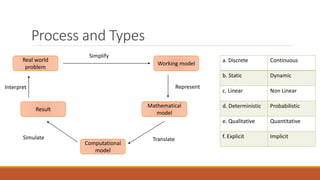
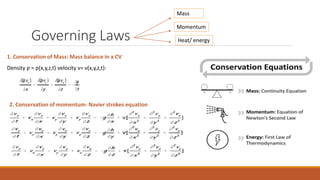
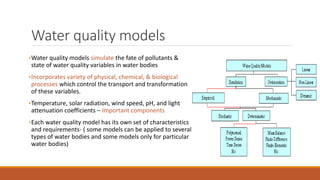
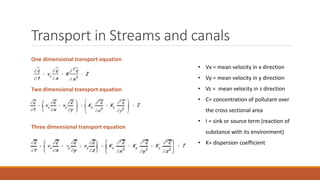
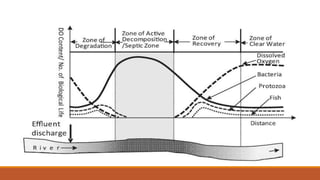
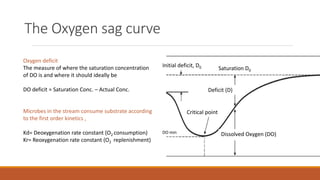
This document provides an overview of mathematical modelling of streams. It discusses the need for modelling to simulate different water quality scenarios and management strategies. It introduces various types of mathematical models and describes the governing laws and equations used in water quality models. Key aspects covered include modelling of dissolved oxygen levels using the Streeter-Phelps model, and a case study applying the QUAL2Kw model to a river in Karnataka, India.










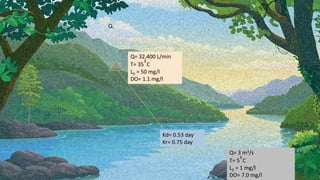
![Q1 What will be the temperature of mixed water ?
T3 = T1Q1 + T2Q2 = (5 ͦͦC)(3 m3/s)+(35 ͦͦC)(0.54m3/s) = 9.6 ͦͦC = 10 ͦͦC
Q3 3.54 m3/s
Q2 What is the DO concentration of mixed water ?
D3 = D1Q1 + D2Q2 = (7.0 mg/l )(3 m3/s)+(1.1 mg/l )(0.54m3/s) = 6.1mg/l
Q3 3.54 m3/s
Q3 What is the time to the critical point of lowest Dissolved oxygen ?
= 1 ln [ 0.75 x (1- 5.17 (0.75-0.53)] = 0.25 day
(0.75-0.53) 0.53 0.53x 8.47](https://image.slidesharecdn.com/mathematicalmodel-221114071951-625ca543/85/mathematical-model-pptx-19-320.jpg)