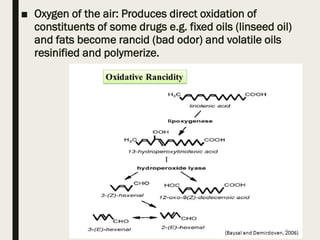
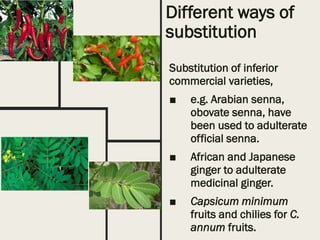
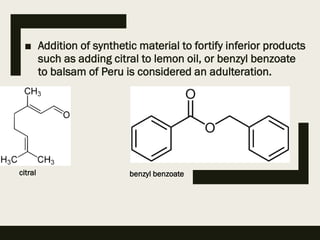
This document discusses the storage, preservation, and evaluation of crude drugs. It notes that drugs can deteriorate during storage due to physicochemical factors like moisture, heat, light, and oxygen or biological factors like fungi, bacteria, insects and rodents. Proper storage involves keeping drugs in an airtight, light-proof container at low temperature. Adulteration of drugs is also discussed, where inferior substances are added to increase weight or substitute for the genuine drug. Evaluation of drugs involves identification and determining quality and purity using organoleptic, microscopic, chemical and other methods.