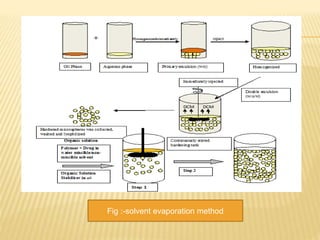
This document discusses magnetic microspheres as a novel drug delivery system, highlighting their characteristics, advantages, and disadvantages. It details various methods of preparation, evaluation techniques for the microspheres, and the impact of magnetic targeting on drug delivery. The document emphasizes the potential benefits of improved therapeutic effects and patient compliance while noting the challenges such as costs and monitoring complexity.