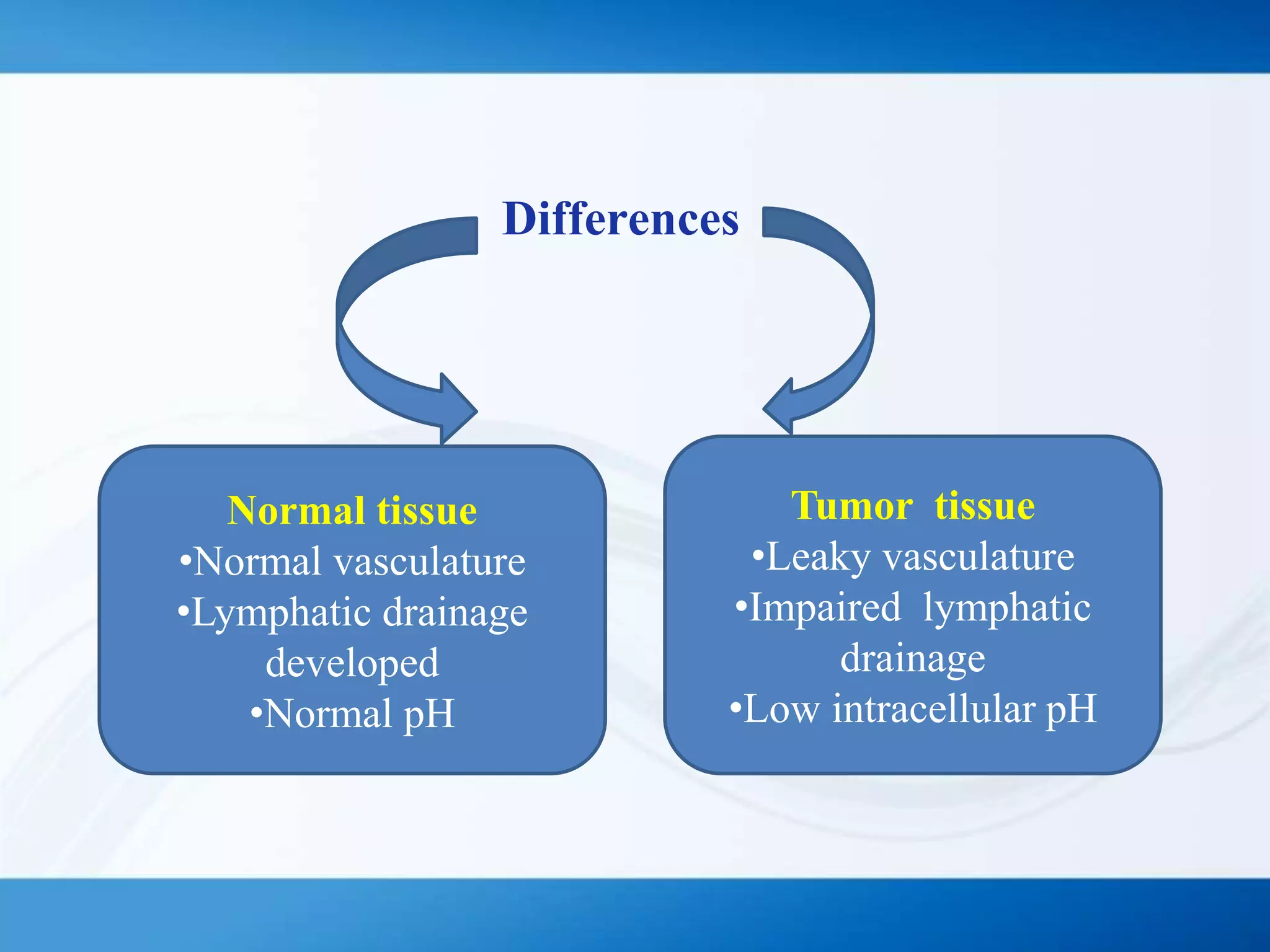
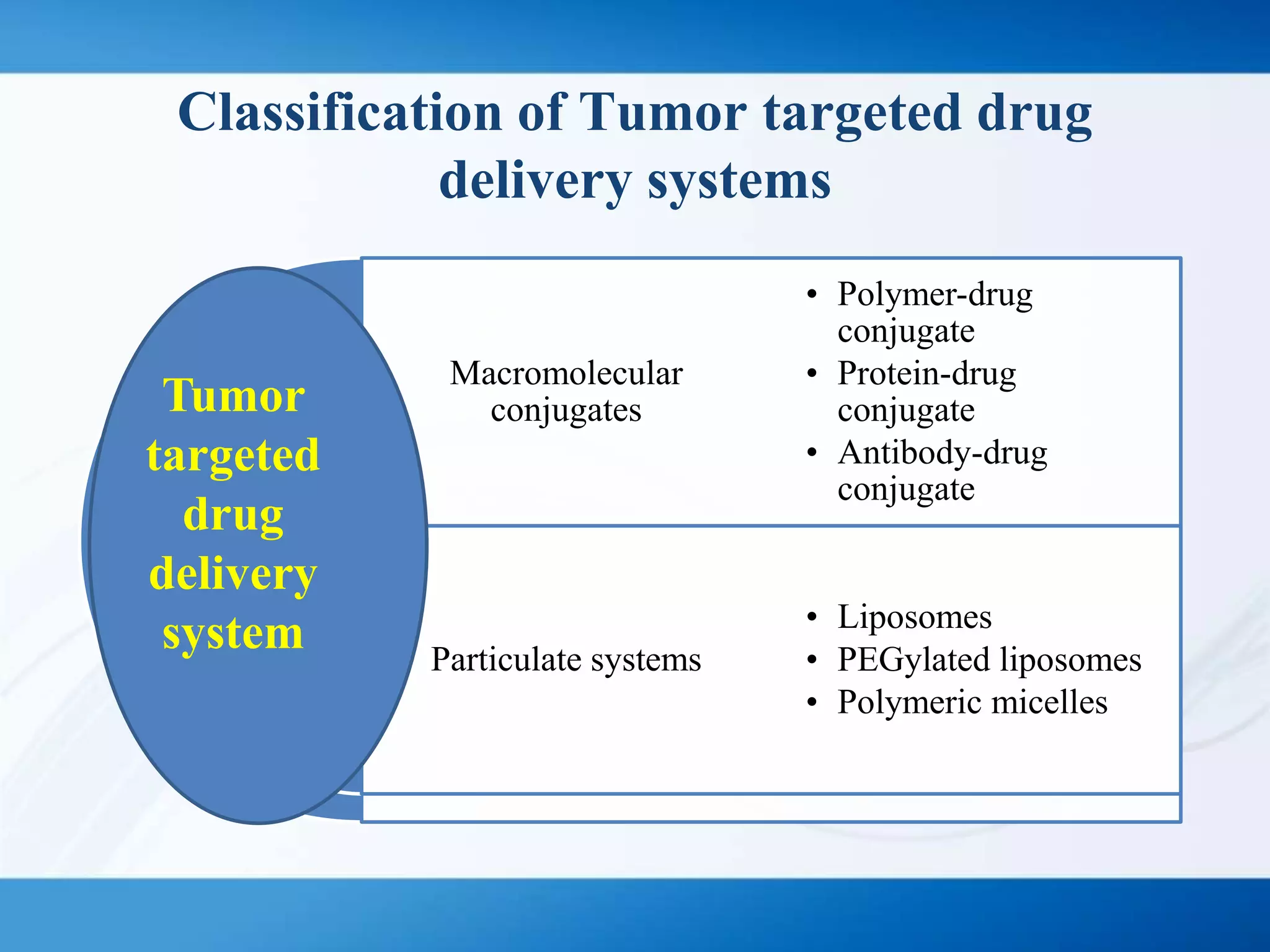
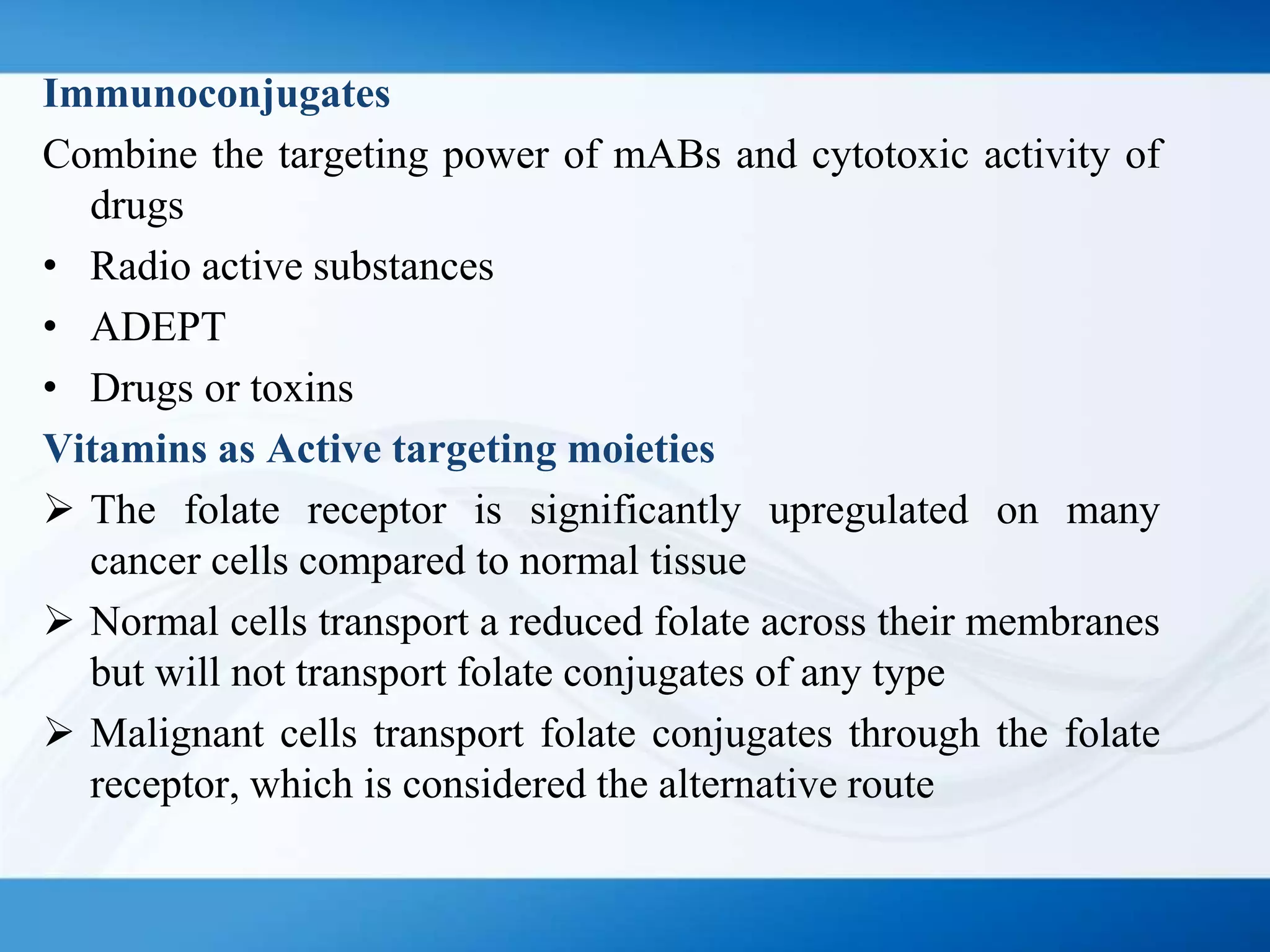
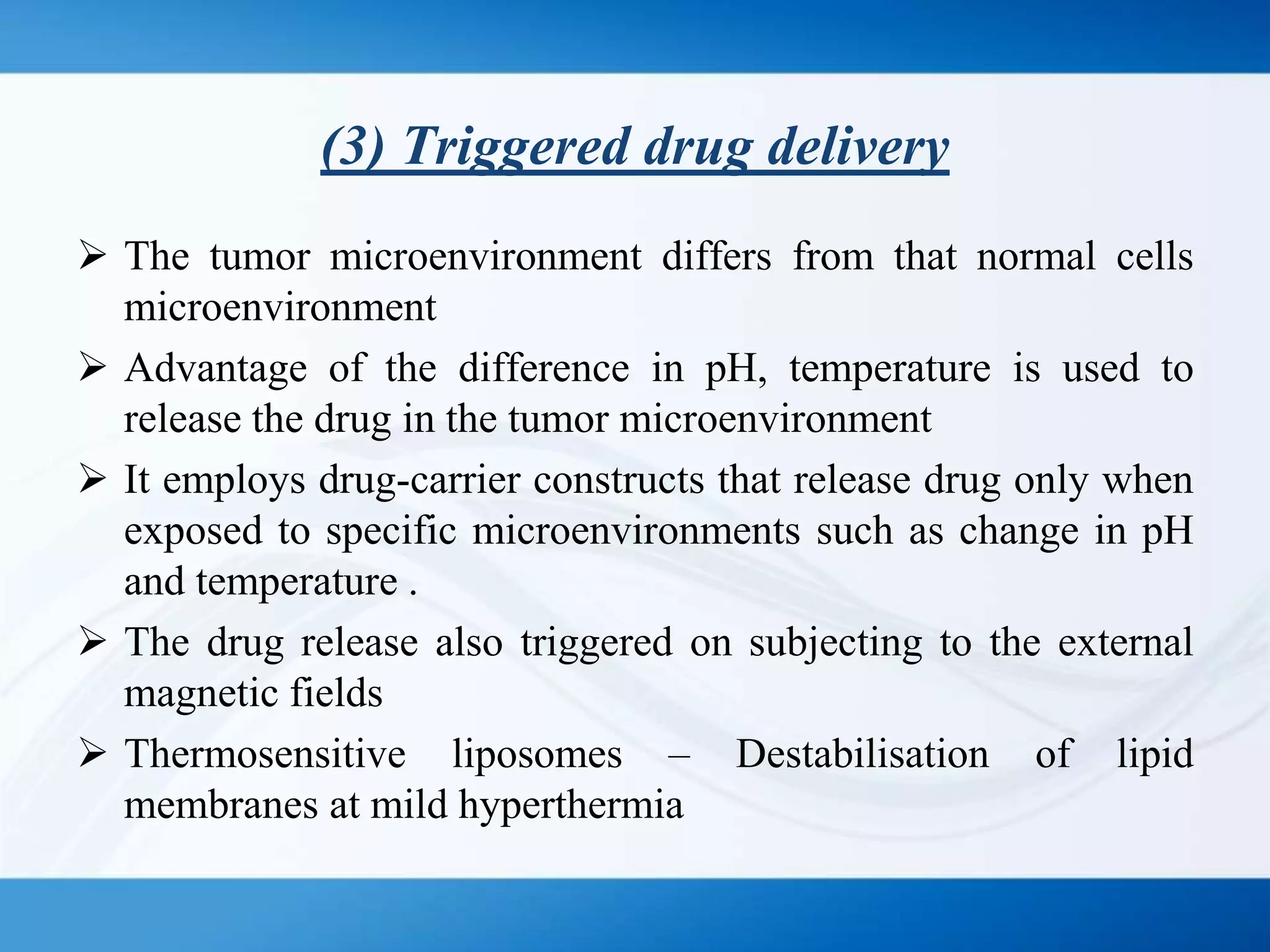
The document discusses tumor targeting in cancer treatment, highlighting the uncontrolled cell division that characterizes cancer and the various types of tumors. It outlines the classification of tumors, their monitoring methods, and principles of tumor targeting including passive and active targeting strategies. Additionally, it covers the development of targeted drug delivery systems and examples of marketed products within this context.