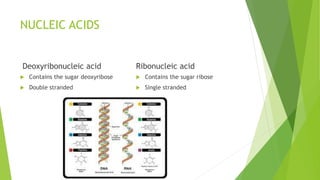
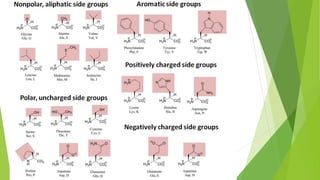
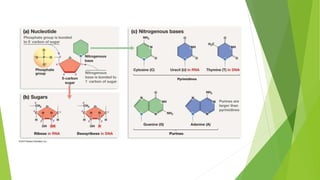
This document defines and describes biomolecules, which are molecules found in living organisms. Biomolecules are divided into macromolecules and micromolecules. Macromolecules have a molecular weight over 1000 and include polysaccharides, nucleic acids, and proteins. Micromolecules have a molecular weight under 1000 and include amino acids, sugars, nucleotides, and lipids. The document then provides examples and further descriptions of these classes of biomolecules, including their structures, functions, and examples.