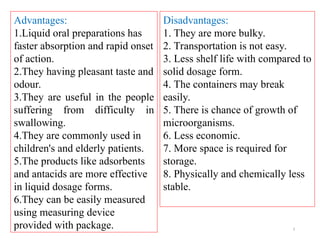
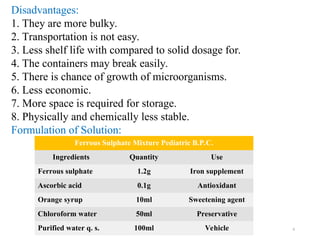
The document discusses liquid oral preparations, including their types, advantages, and disadvantages, emphasizing the faster absorption and pleasant taste of solutions, syrups, and elixirs. It describes formulation and manufacturing considerations, as well as quality control tests for liquid orals, highlighting aspects like clarity, viscosity, and microbial limit tests. Additionally, it provides a specific formulation example for a pediatric iron supplement solution.