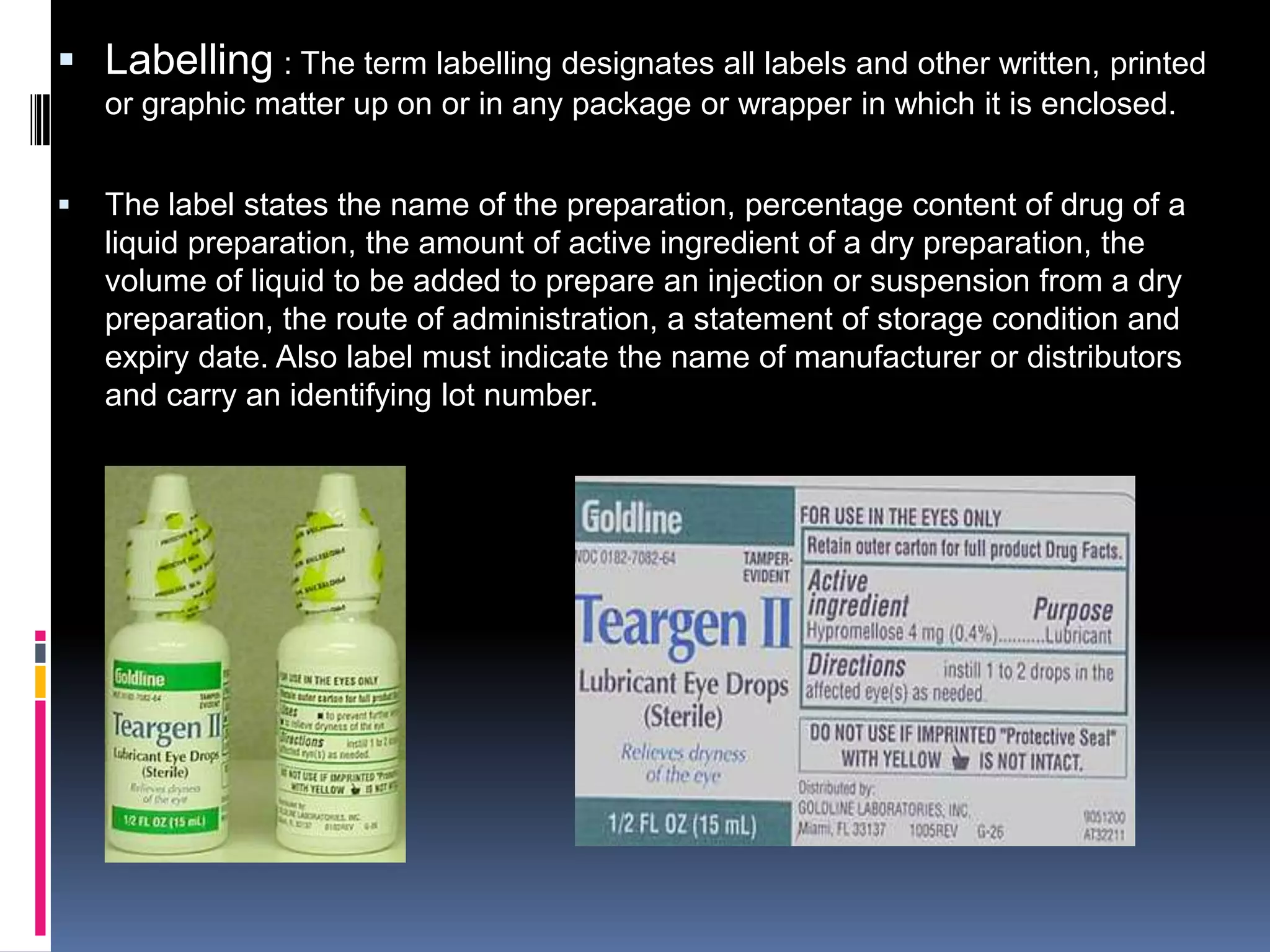
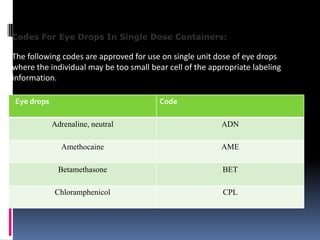
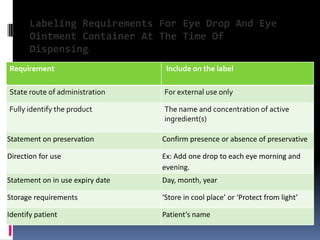
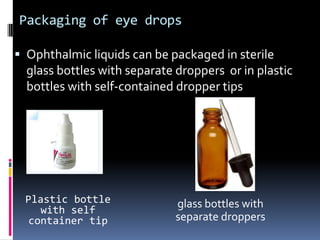
The document discusses labeling requirements and packaging for ophthalmic preparations. It states that labels must include the name of the drug, dosage, manufacturer, and expiration date. Labels can be printed directly on containers or applied separately. Eye drop packaging comes in glass bottles with droppers or plastic bottles with integrated droppers. Plastic is now more common. Packaging must be sterile and can be single-dose without preservatives or multi-dose which requires preservatives to maintain sterility between uses.