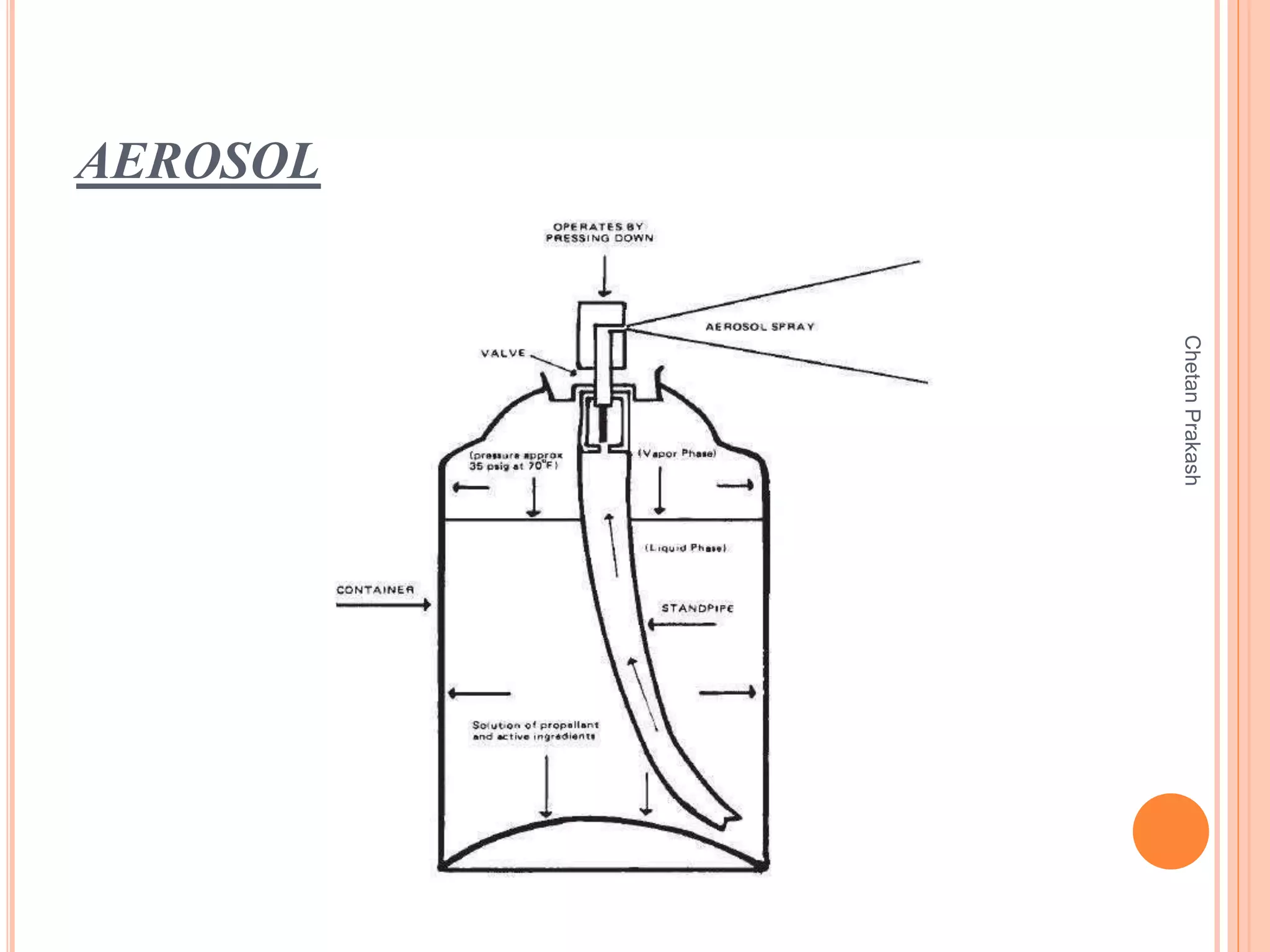
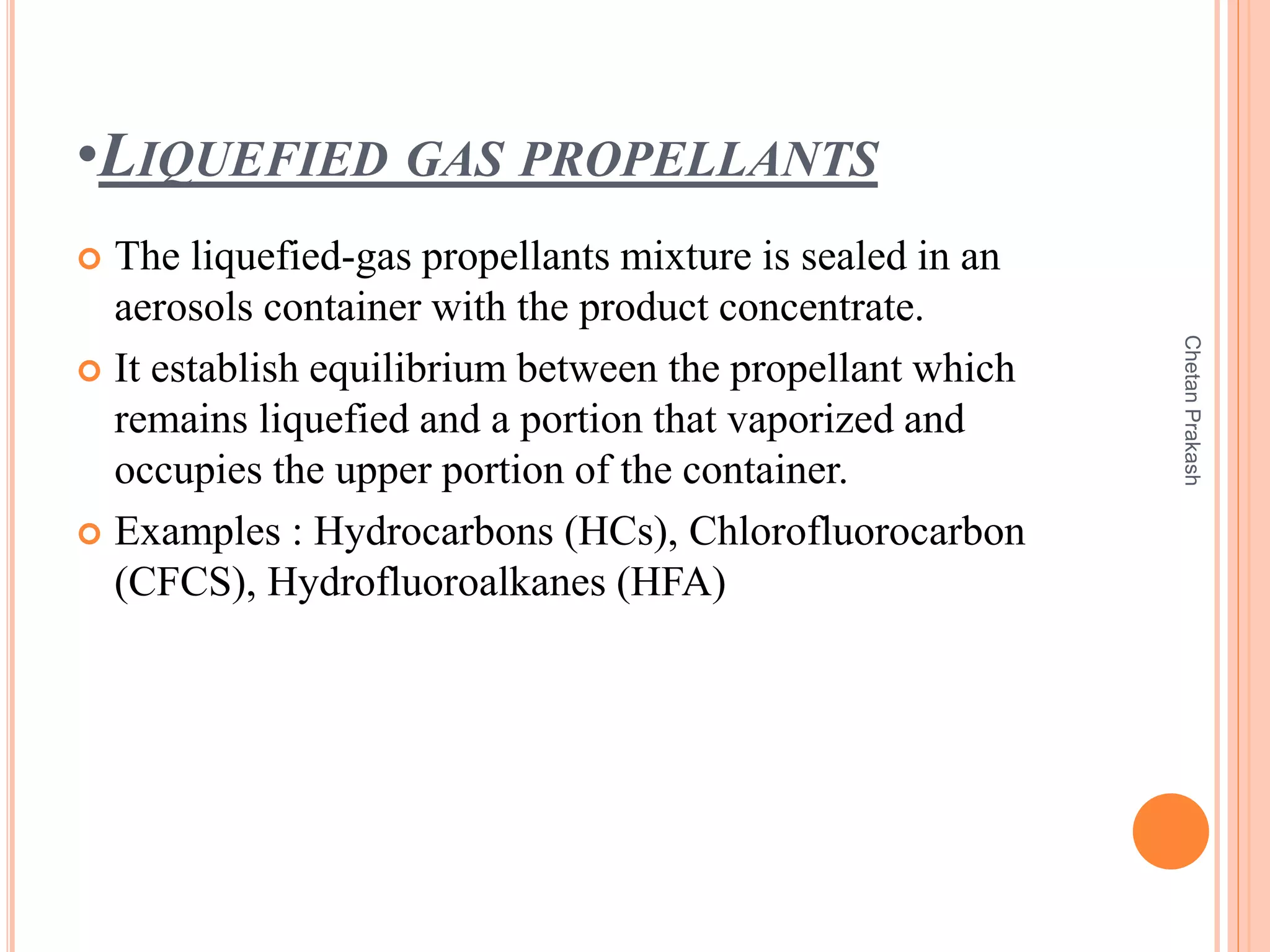
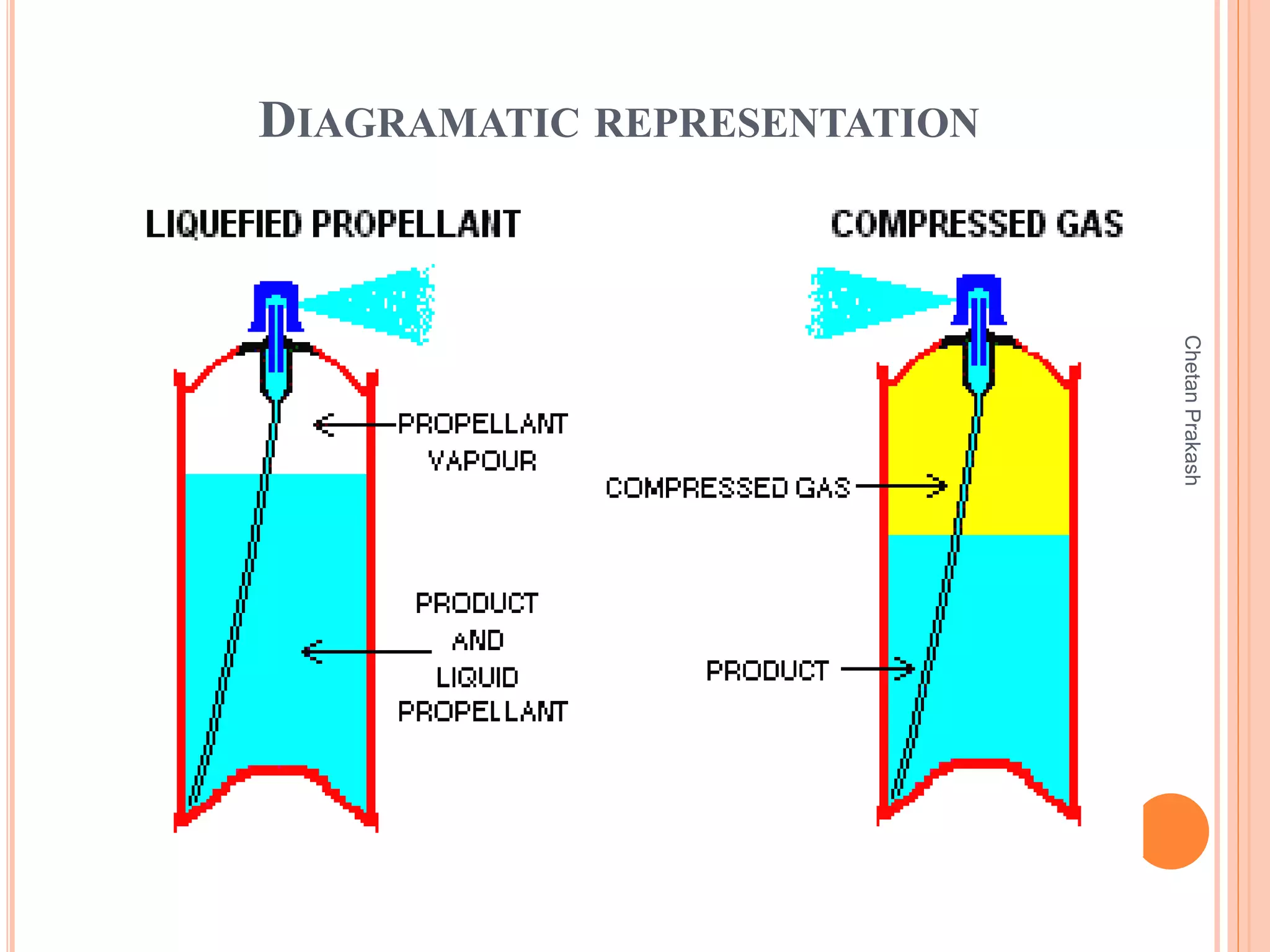
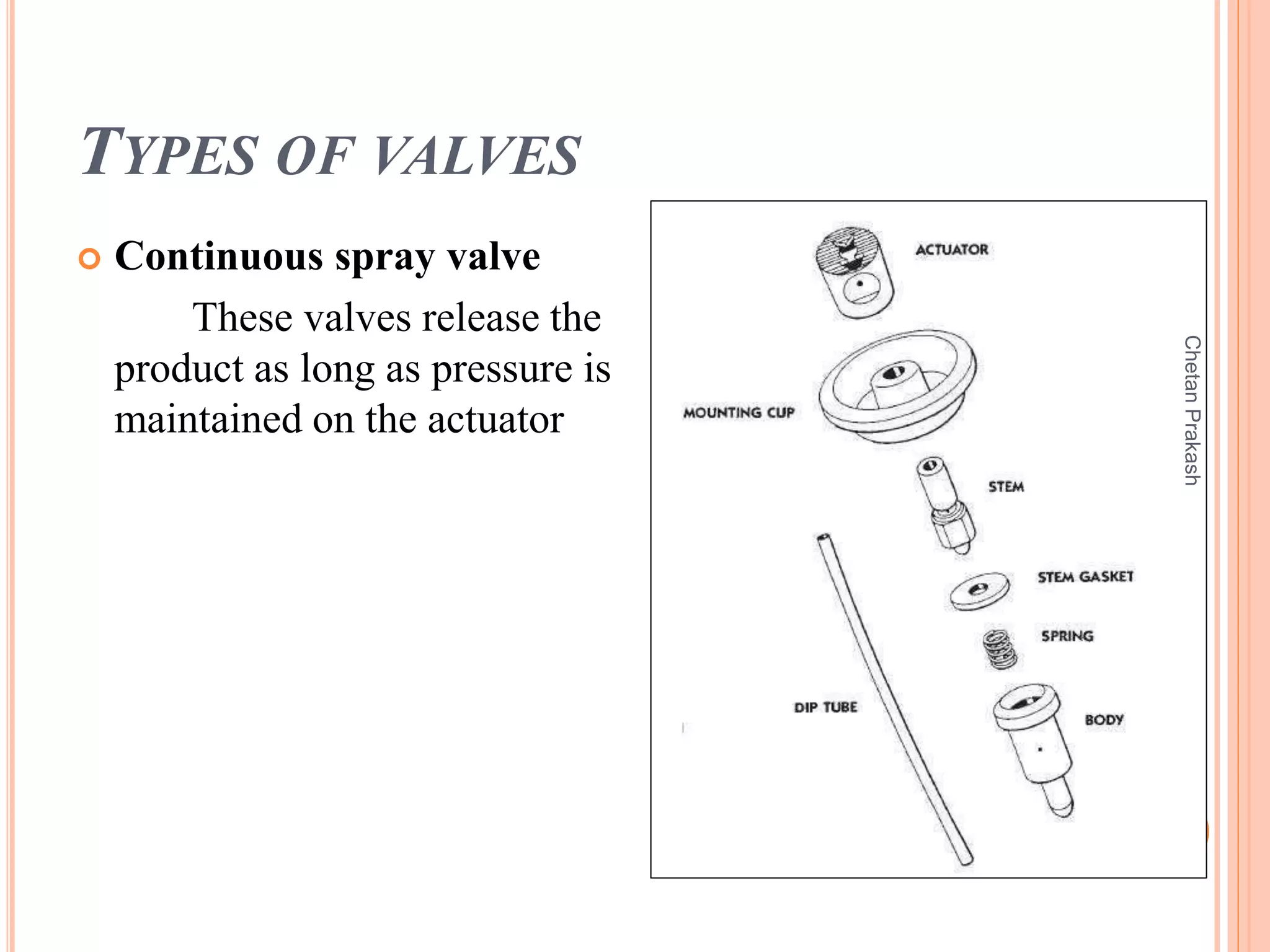
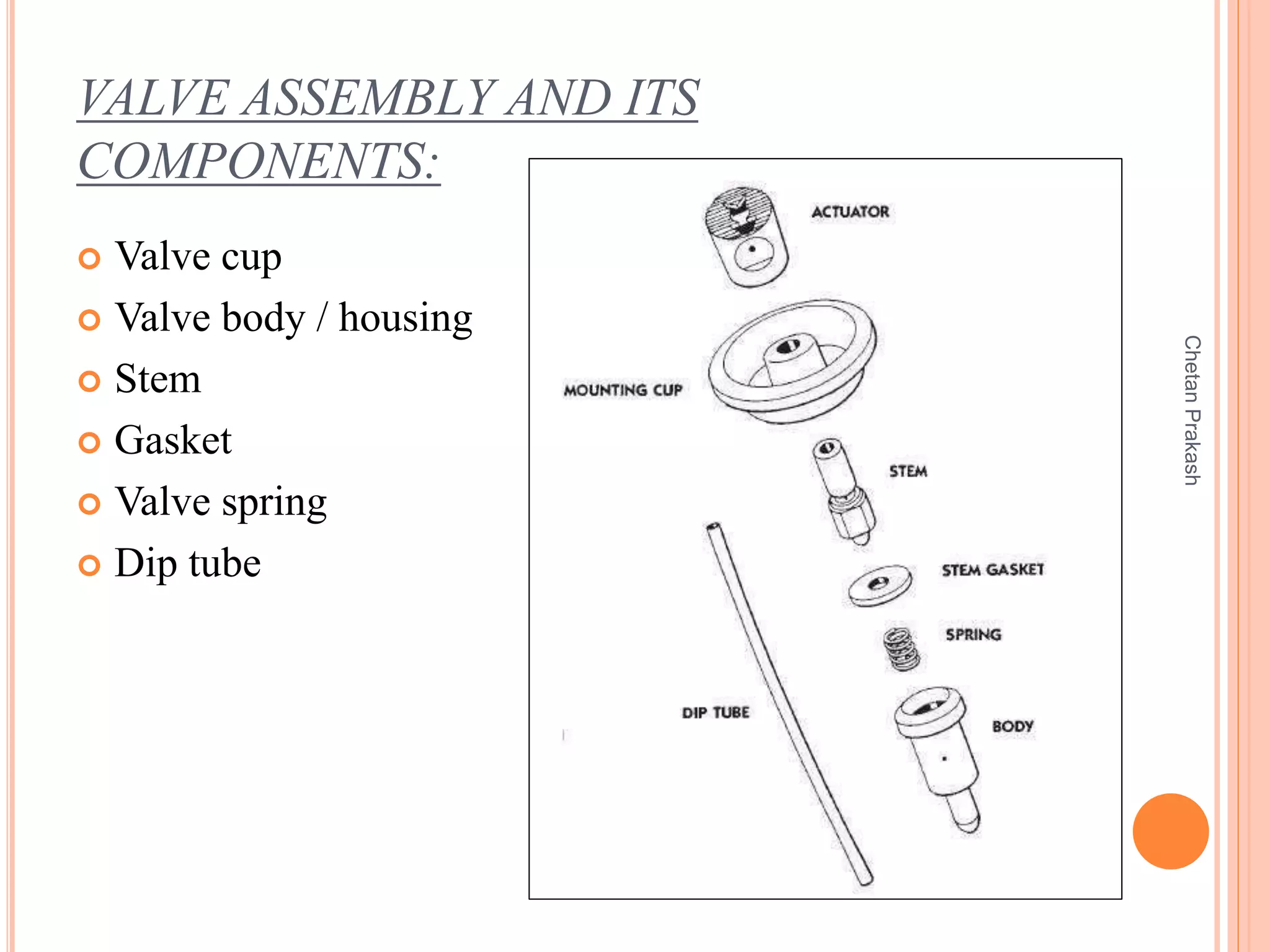
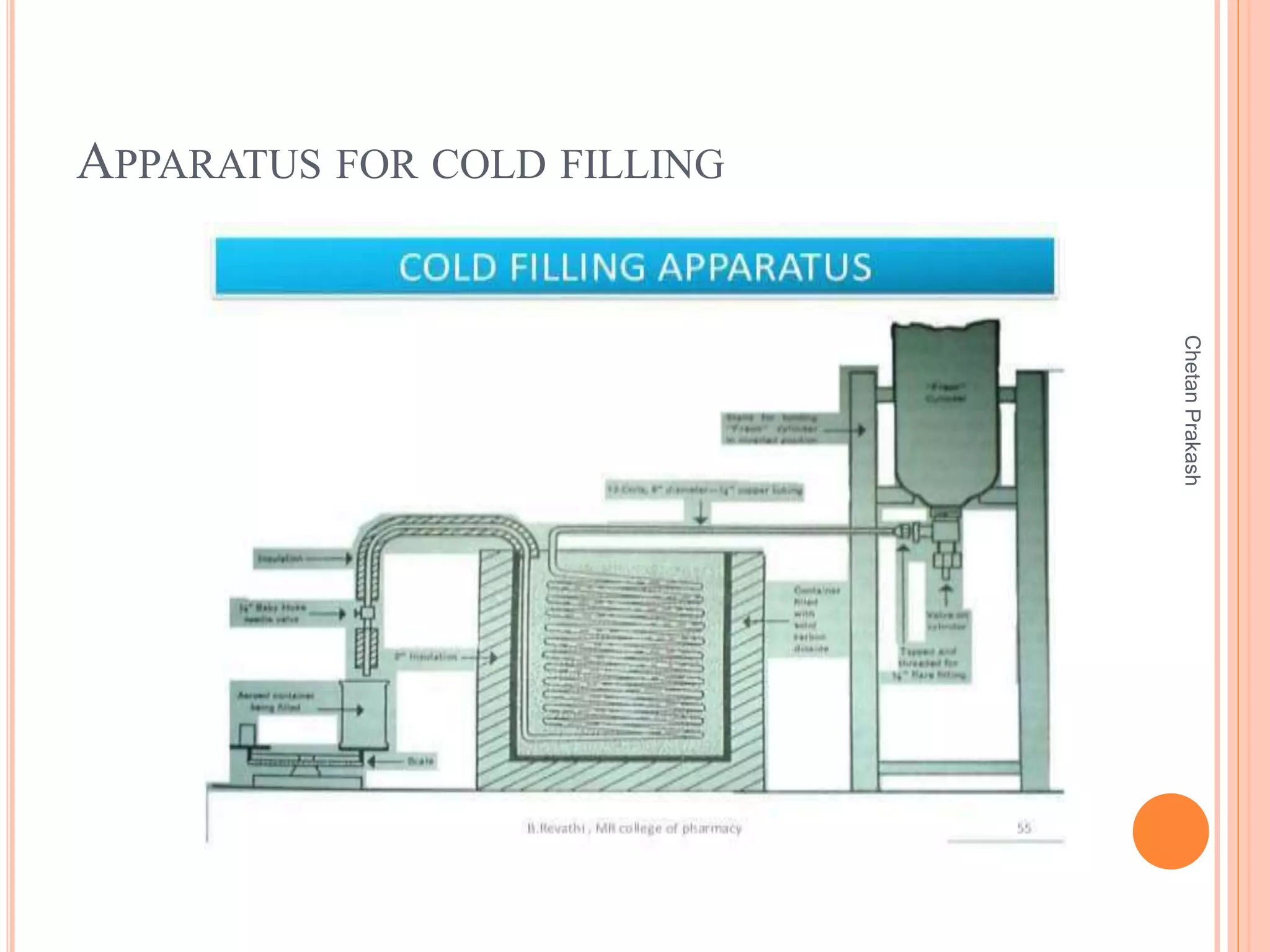
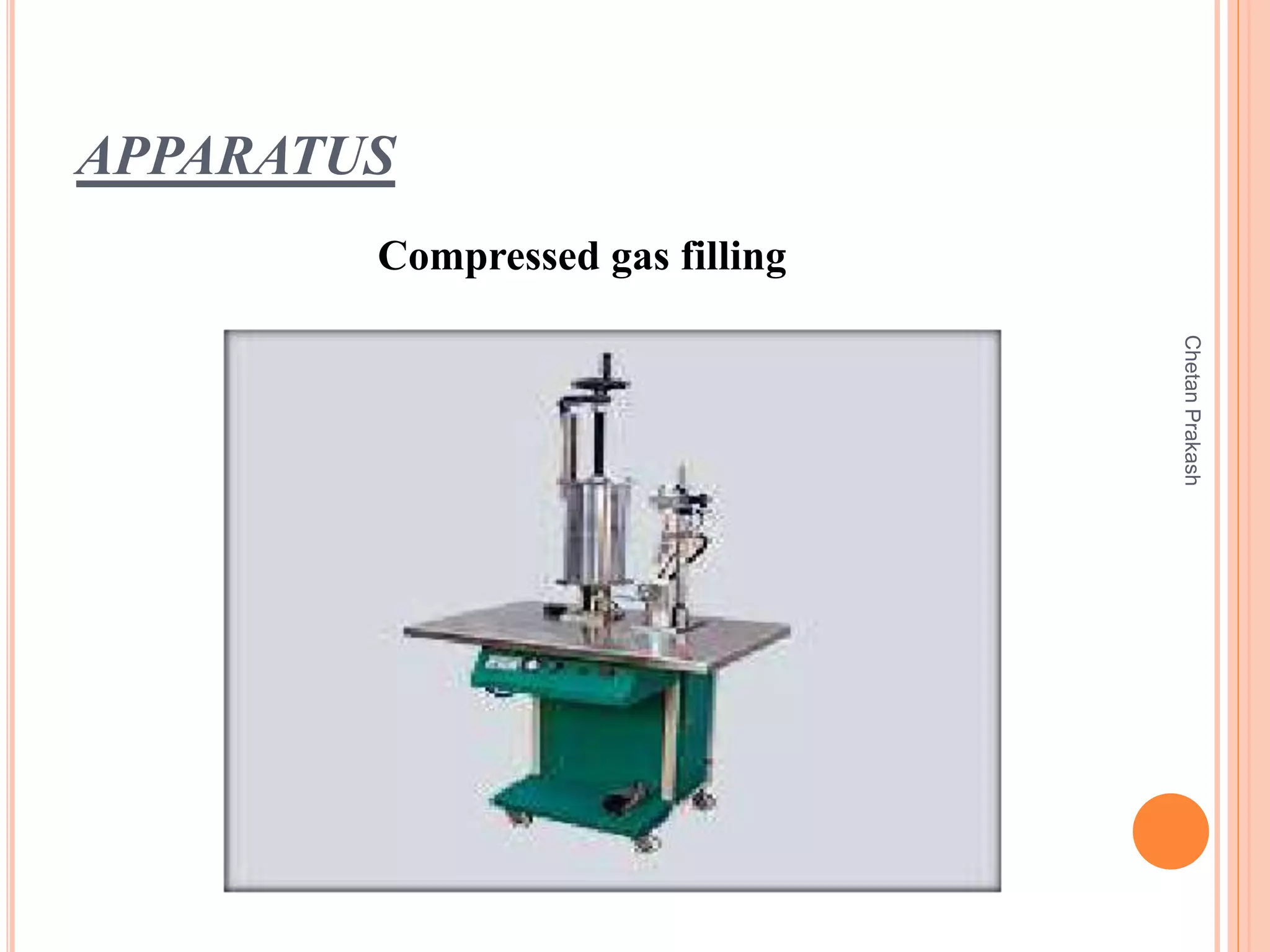
This document provides a comprehensive overview of aerosol containers used in pharmaceutical and cosmetic applications, detailing their components, types of propellants, advantages, disadvantages, and manufacturing processes. It highlights key features such as the role of valves and actuators, as well as various aerosol systems and their evaluation through physical and biological tests. Additionally, it discusses the importance of proper packaging, labeling, and storage methodologies to ensure product efficacy and safety.