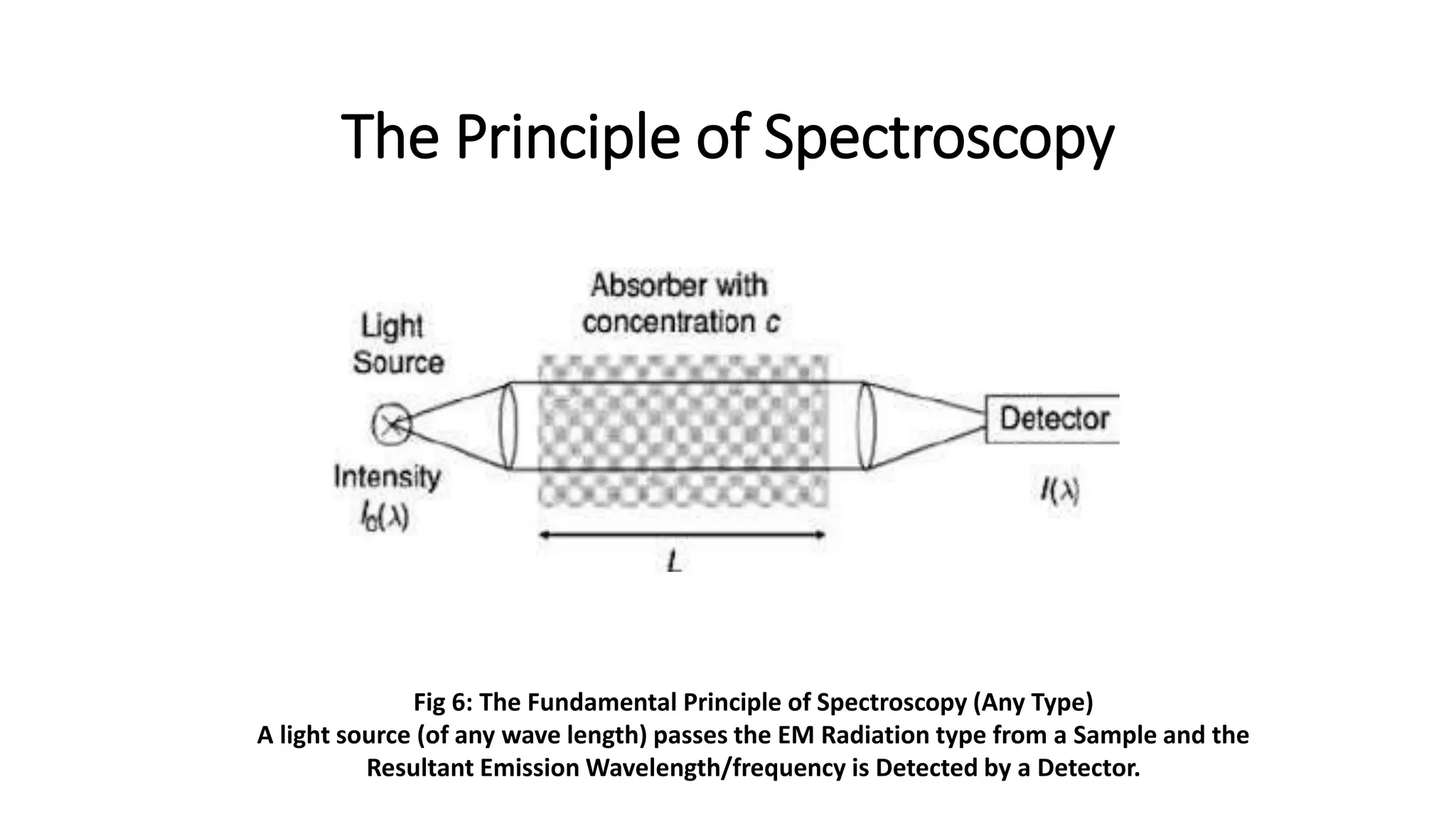
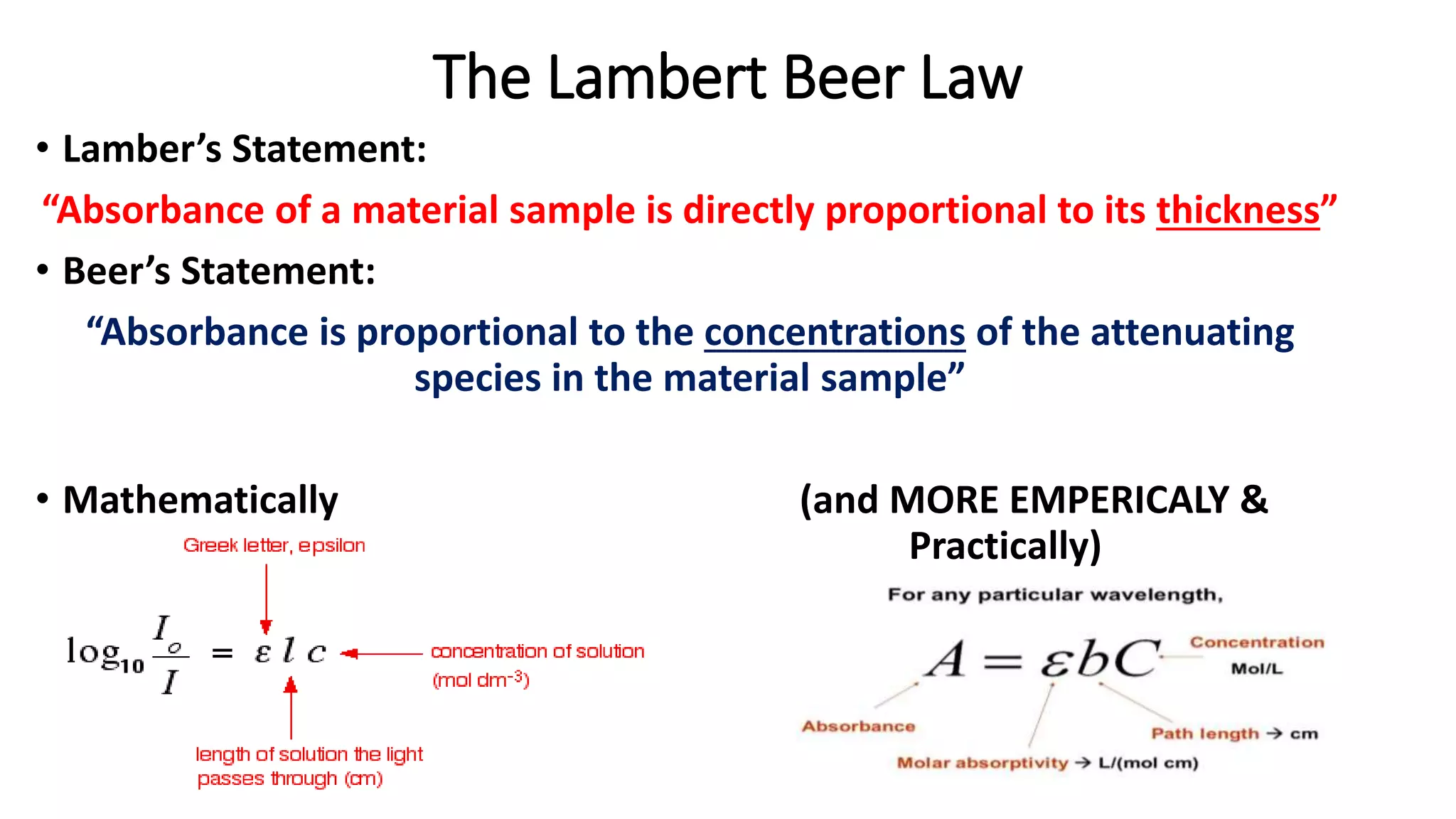
This document provides an introduction to spectroscopy, explaining the electromagnetic spectrum and the interaction of light with matter. It covers key concepts such as ionizing radiation, wave energy equations, and the principles behind various types of spectroscopy including UV-visible and NMR spectroscopy. Additionally, it discusses foundational figures in the field and includes mathematical laws relevant to absorbance.



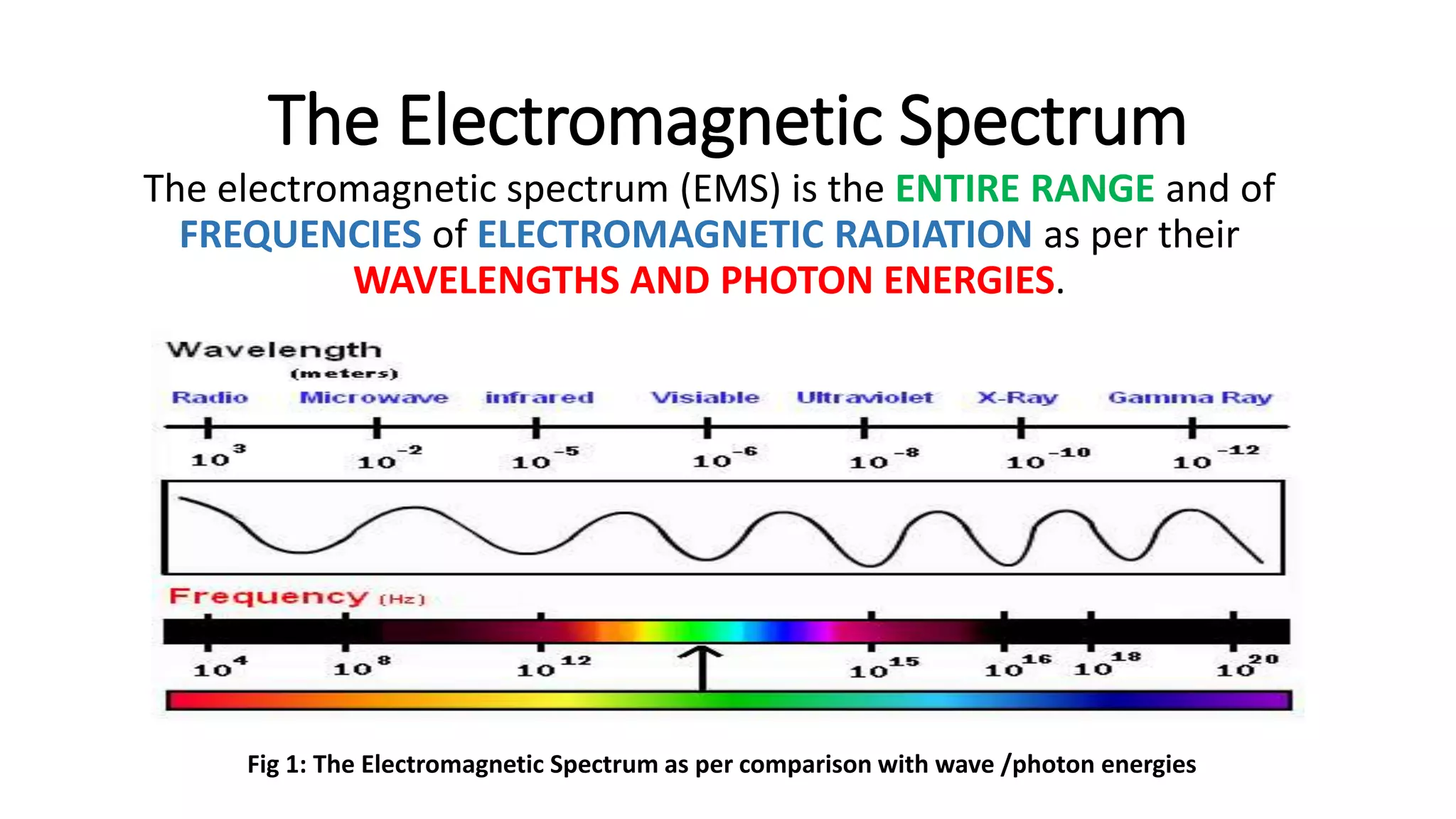





![The Wave Energy Equation (Plank’s Equation)
E=h.ʋ
Here:
E: Energy
h: Plank’s Constant (6.63x10-34 J.s)
ʋ: Frequency of a wave [f= 1/(c/ƛ)] or [ f= 1/t(sec))](https://image.slidesharecdn.com/introductiontospectroscopy-171004065530/75/Introduction-to-spectroscopy-10-2048.jpg)