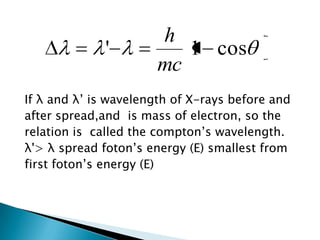Embed presentation
Downloaded 23 times

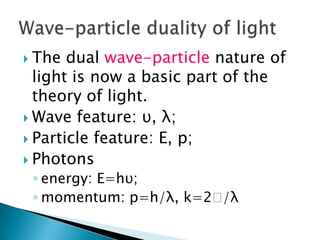
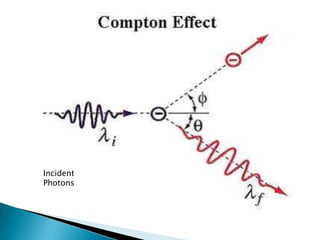
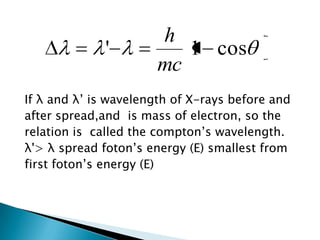



The document discusses the dual wave-particle nature of light and photons. It explains that light exhibits both wave features like frequency and wavelength, as well as particle features like energy and momentum. The document also discusses Compton scattering, where photons transfer some of their energy to electrons, increasing their wavelength. Additionally, it covers de Broglie's proposal that matter has both wave and particle properties, and introduces the concept of de Broglie wavelength for particles like electrons.