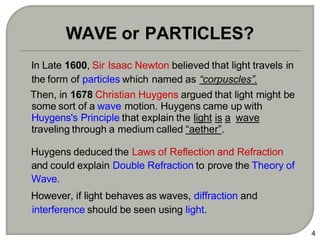
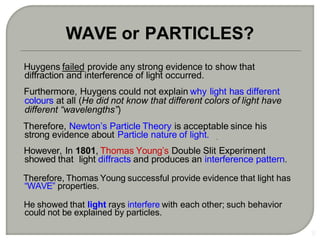
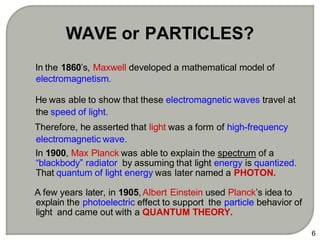
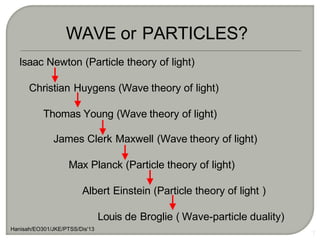
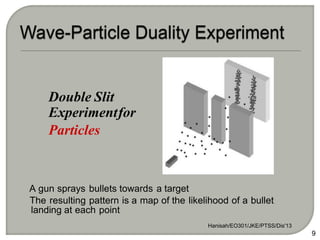
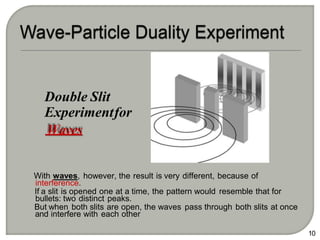
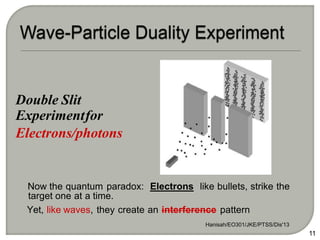
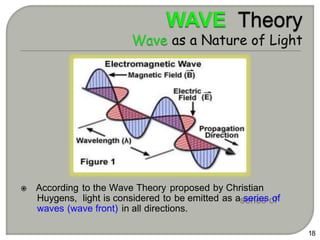
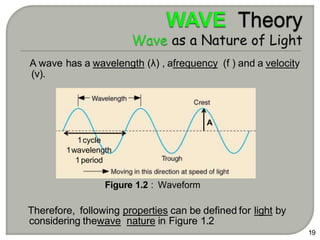
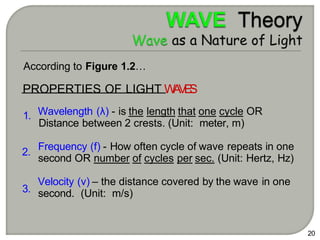
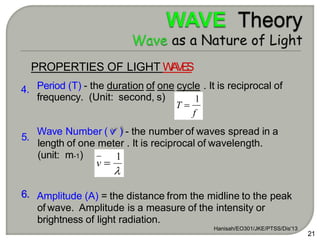
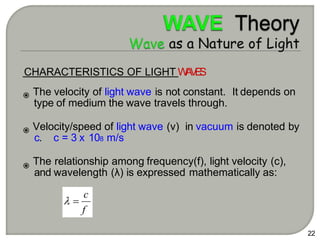
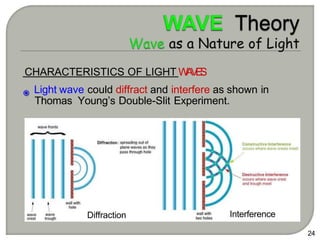
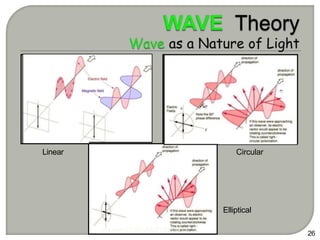
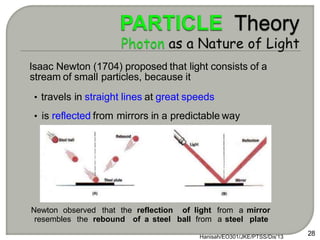
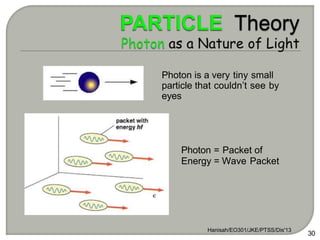
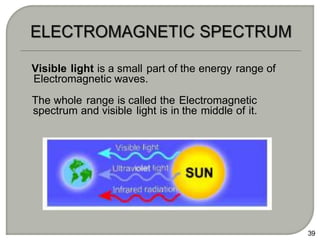
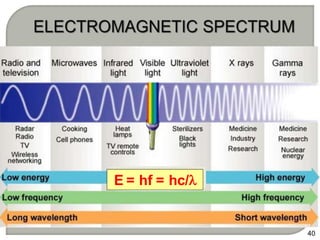
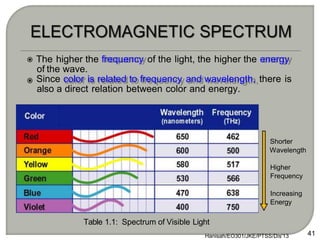
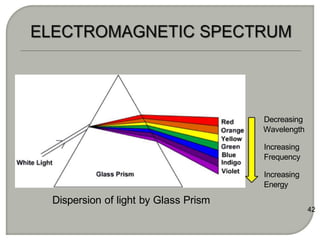
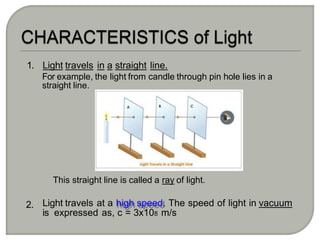
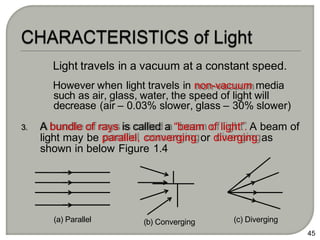
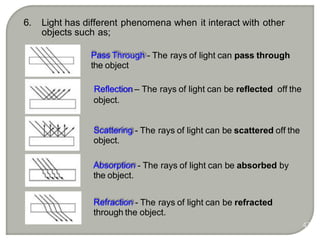
Light can behave as both a wave and a particle. For centuries, scientists debated the nature of light, with some arguing it was a wave and others arguing it was a particle. Through experiments like the double slit experiment, it became clear that light exhibits properties of both waves and particles. The wave-particle duality of light is now described by quantum mechanics. Light acts as a wave when it travels and interferes, and as a particle, called a photon, when it is emitted, absorbed, or quantized. Both the wave and particle theories helped explain different observed phenomena but only the modern quantum mechanical theory of light fully describes its dual behavior.