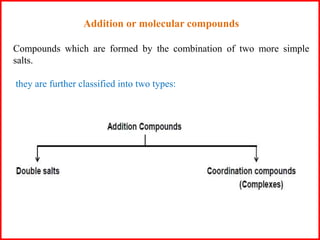
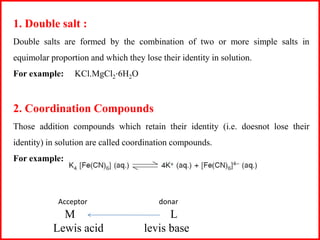
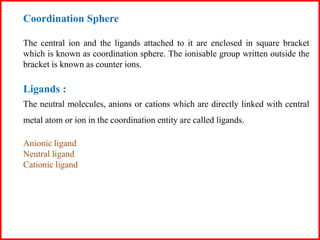
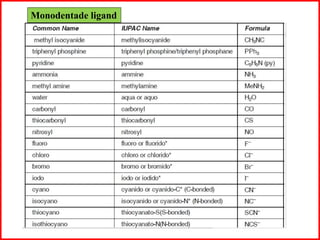
This document discusses types of addition compounds and coordination compounds. It defines double salts as compounds formed from two simple salts in equimolar proportions that lose their identity in solution. Coordination compounds retain their identity in solution. It also defines key terms related to coordination compounds such as central atom/ion, coordination number, coordination sphere, ligands, and provides examples of each. Naming conventions for coordination compounds are also outlined.

![K4[Fe(CN)6]
Coordination no.
Central metal ion
Ligand
Ionisable sphere
Or
counter sphere
Anionic complex
Coordination sphere](https://image.slidesharecdn.com/coordination1-200509044750/85/Coordination-Chemistry-4-320.jpg)
![Central Atom/Ion :
the atom/ion to which are bound a fixed number of ligands in a definite
geometrical arrangement around it, is called the central atom or ion.
For example:
the central atom/ion in the coordination entities: [NiCl2(OH2)4],
[CoCl(NH3)5]2+ and [Fe(CN)6]3+ are Ni2+, Co3+ and Fe3+ Respectively.
Coordination Number :
It is defined as the number of coordinate bonds formed by central metal atom,
with the ligands.](https://image.slidesharecdn.com/coordination1-200509044750/85/Coordination-Chemistry-5-320.jpg)
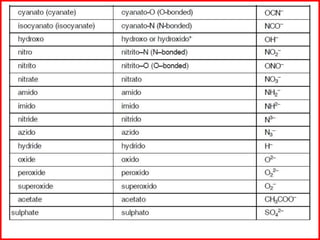
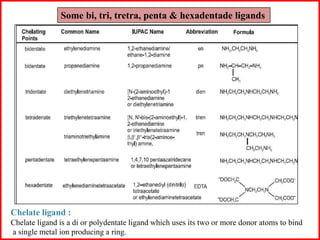
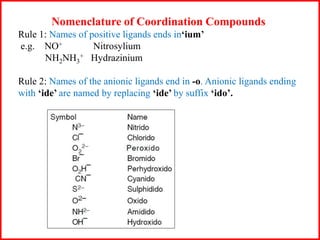
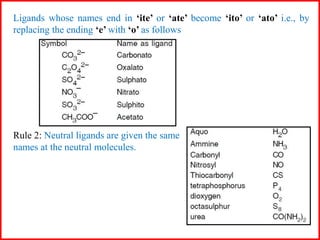
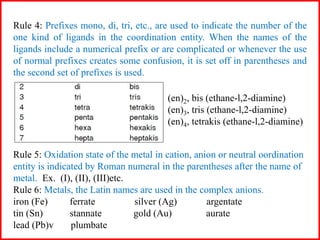
![Some examples are:
(i) [Cr(NH3)3(H2O)3]Cl3 (complex cation + simple anion)
triamminetriaquachromium (III) chloride
(ii) [Co(NH2CH2CH2NH2)3]2(SO4) (complex cation + simple anion)
tris (ethane-l,2-diamine) cobalt (III) sulphate
(iii) K4 [Fe(CN)6] (simple cation + complex anion)
potassium hexacyanoferrate (II)
[NiCl2(PPh3)2]
dichloridobis(triphenylphosphine)nickel(II).
Cations + no. of ligands + name of ligand + central metal ion +
oxidation no. (in roman)(alphabatic order)](https://image.slidesharecdn.com/coordination1-200509044750/85/Coordination-Chemistry-13-320.jpg)
