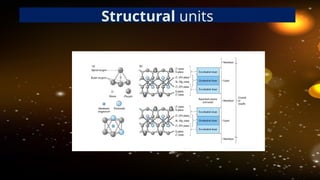
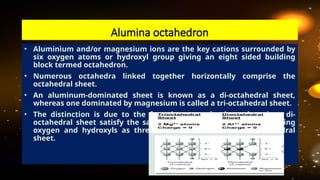
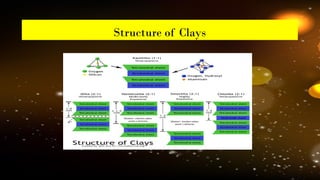
The document discusses the classification and structure of layer lattice silicate clays, also known as phyllosilicates, which consist of tetrahedral and octahedral sheets structured through cations bound by shared oxygen atoms. It differentiates between various types of clays (1:1, 2:1, and 2:1:1) and describes their structural characteristics, including their formation through weathering processes and their interactions with water and cations. Notably, the clay minerals exhibit varying expansiveness based on their compositions, with smectite being highly expanding, while others like kaolinite and illite are less so.