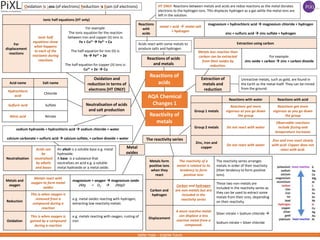
1. The document discusses the reactivity series and how it arranges metals in order of their reactivity based on their tendency to form positive ions.
2. Key reactions include displacement reactions where a more reactive metal can displace a less reactive one from a compound. Metals also react with oxygen to form metal oxides.
3. Extraction of metals using carbon and electrolysis is covered, where metals less reactive than carbon can be extracted from their oxides by reduction, and how electrolysis uses electricity to extract metals that are too reactive to be extracted by carbon.