This document provides an overview of energy topics including:
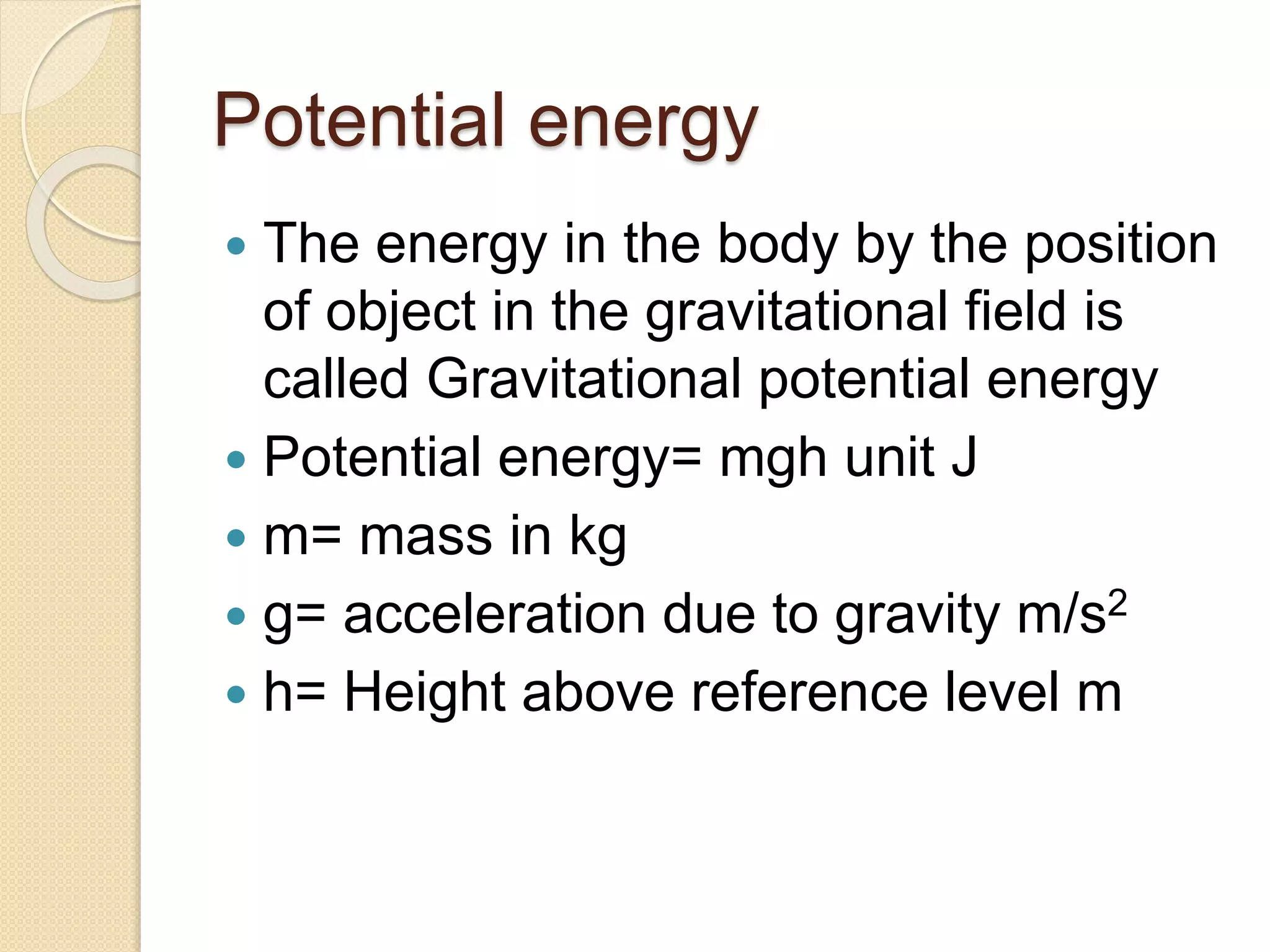
- Definitions of key energy terms like joules, watts, kinetic energy, and potential energy.
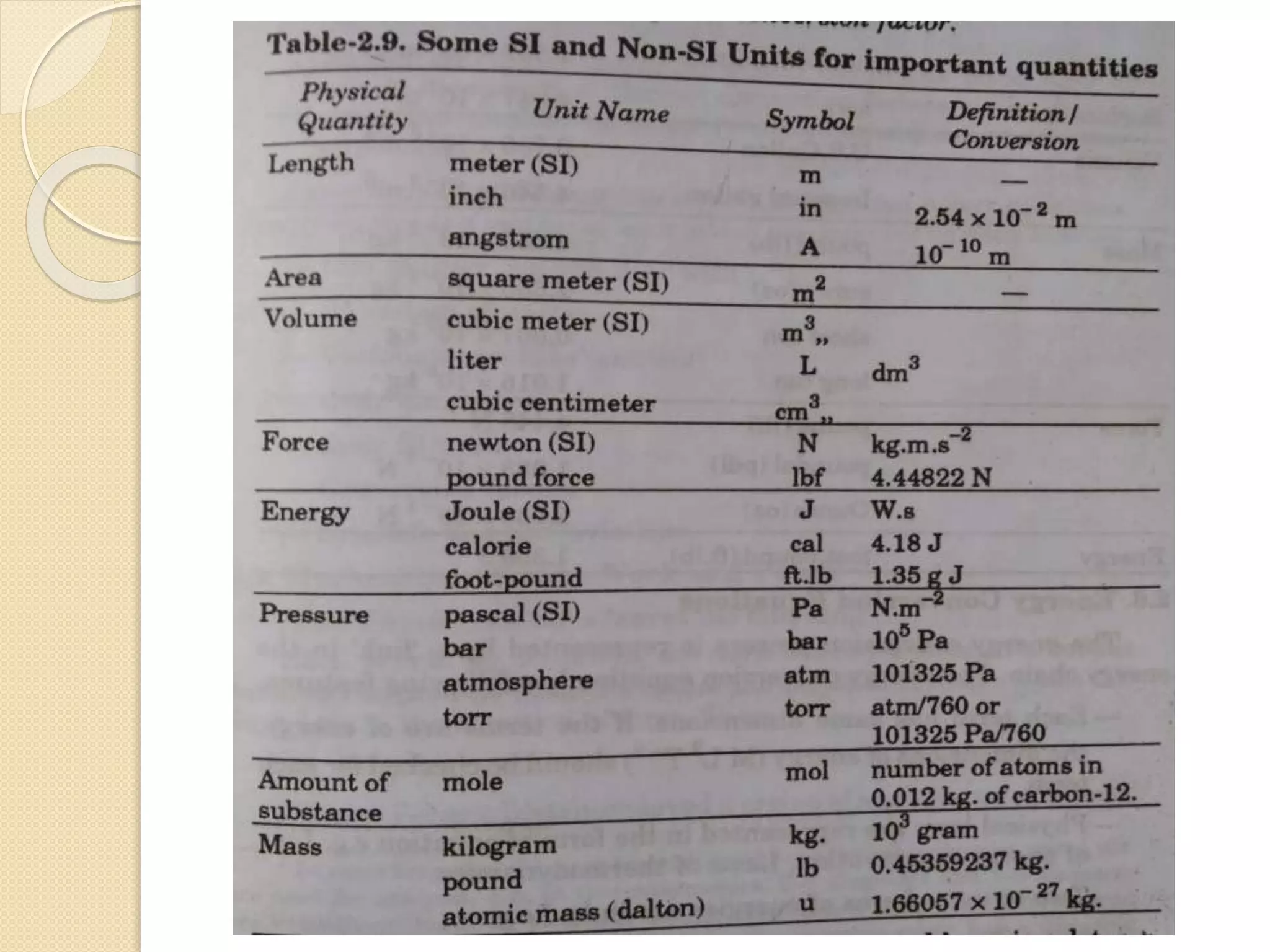
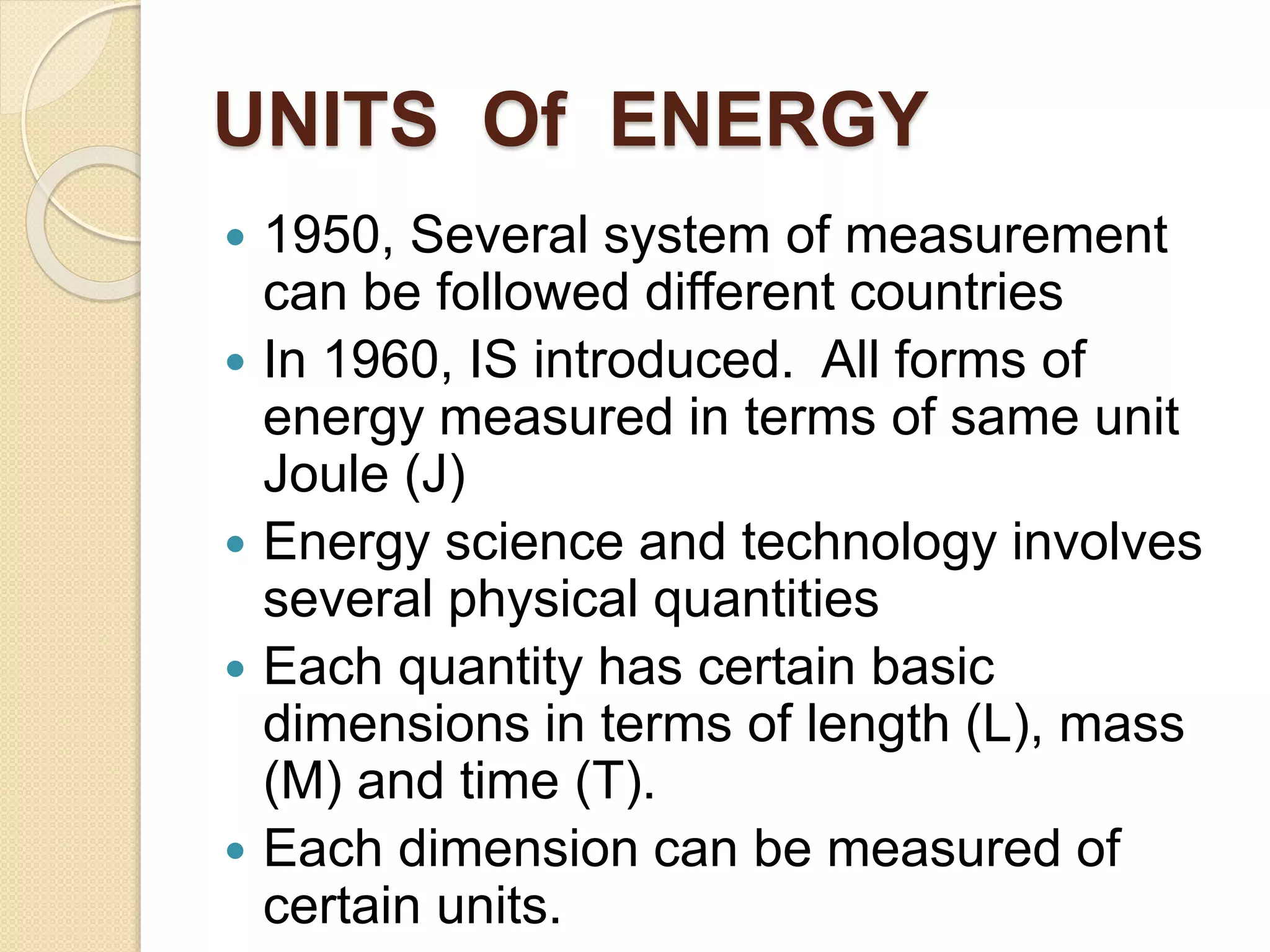
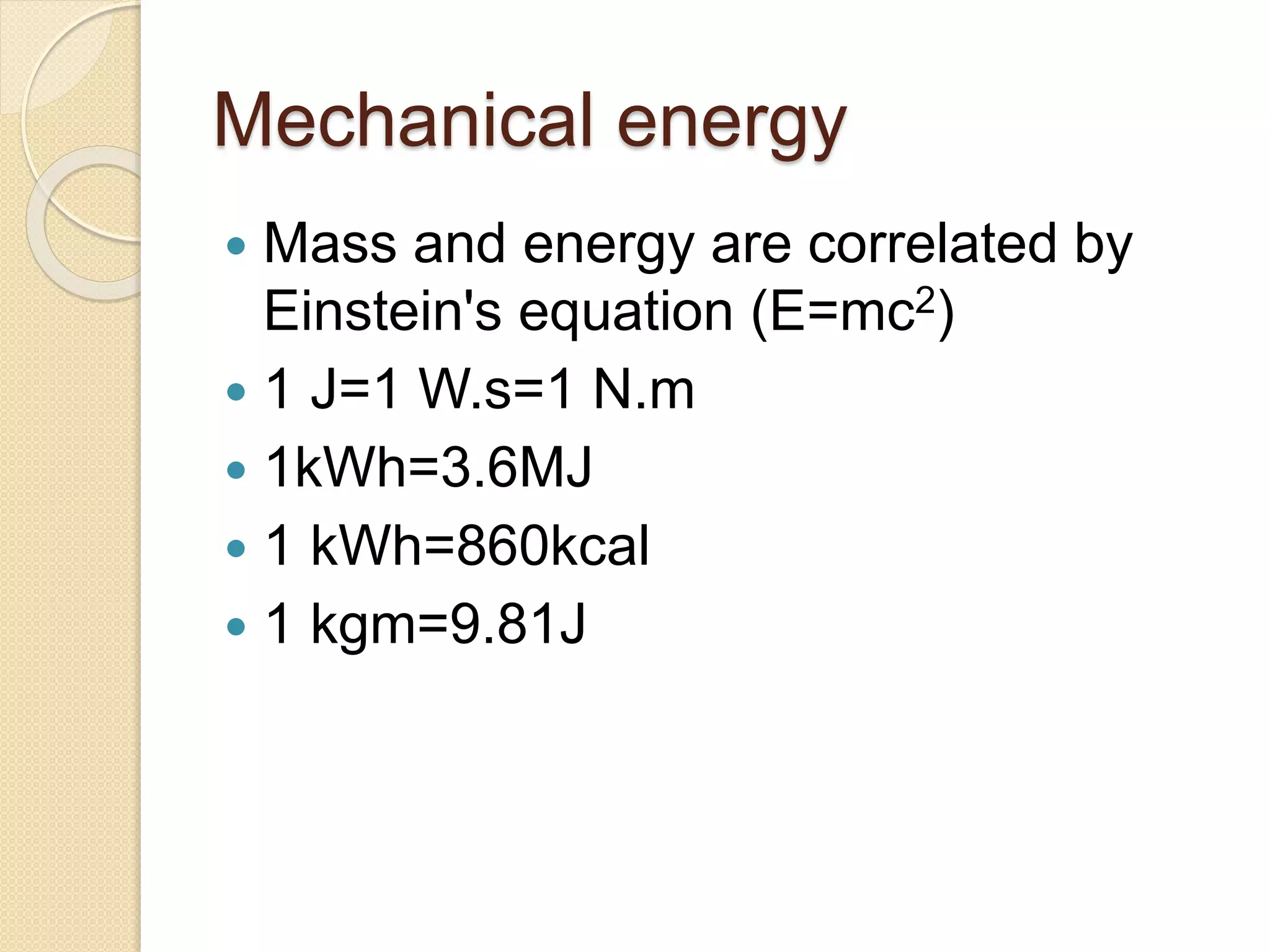
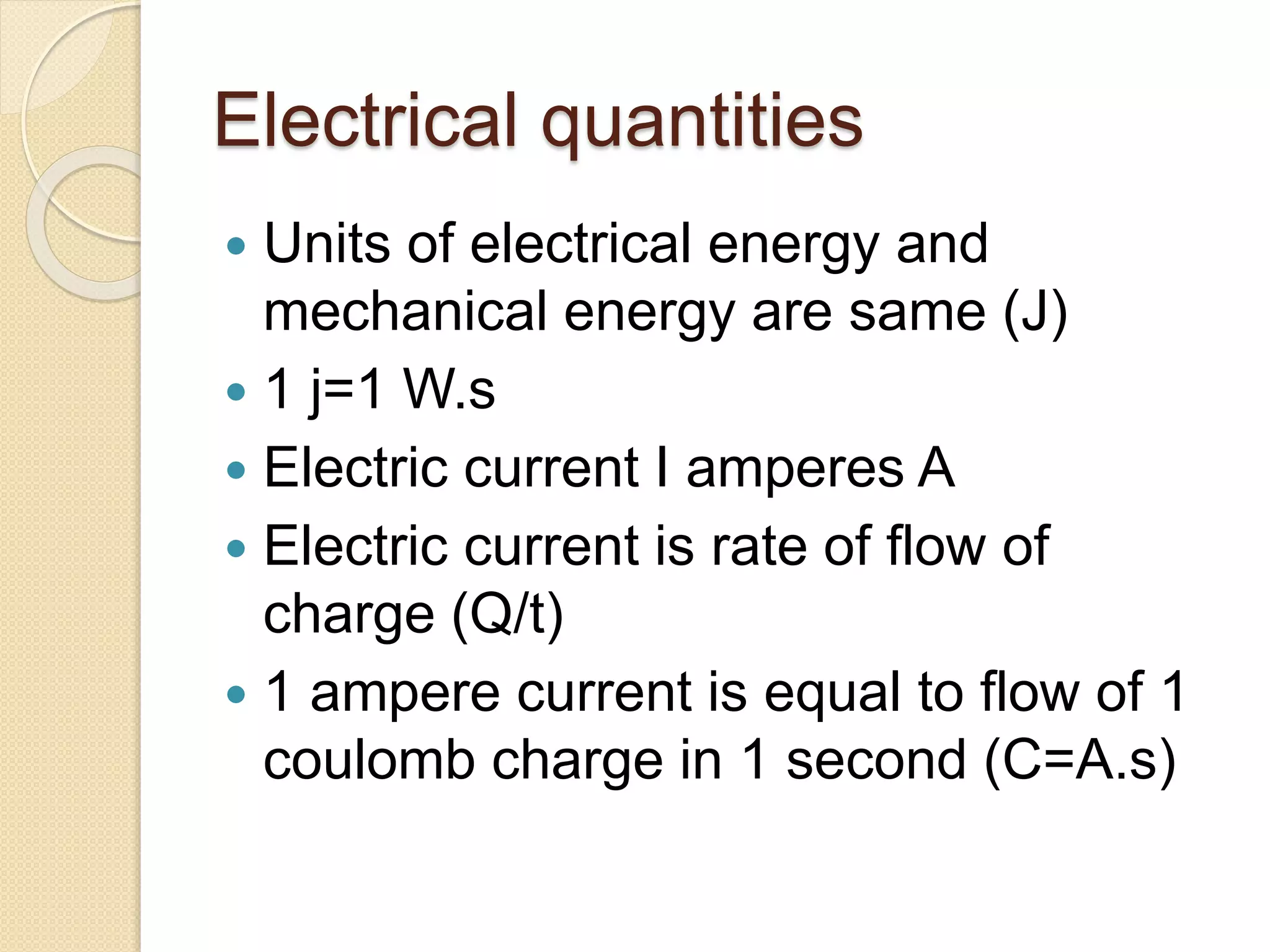
- Explanations of conversion factors between different energy units.
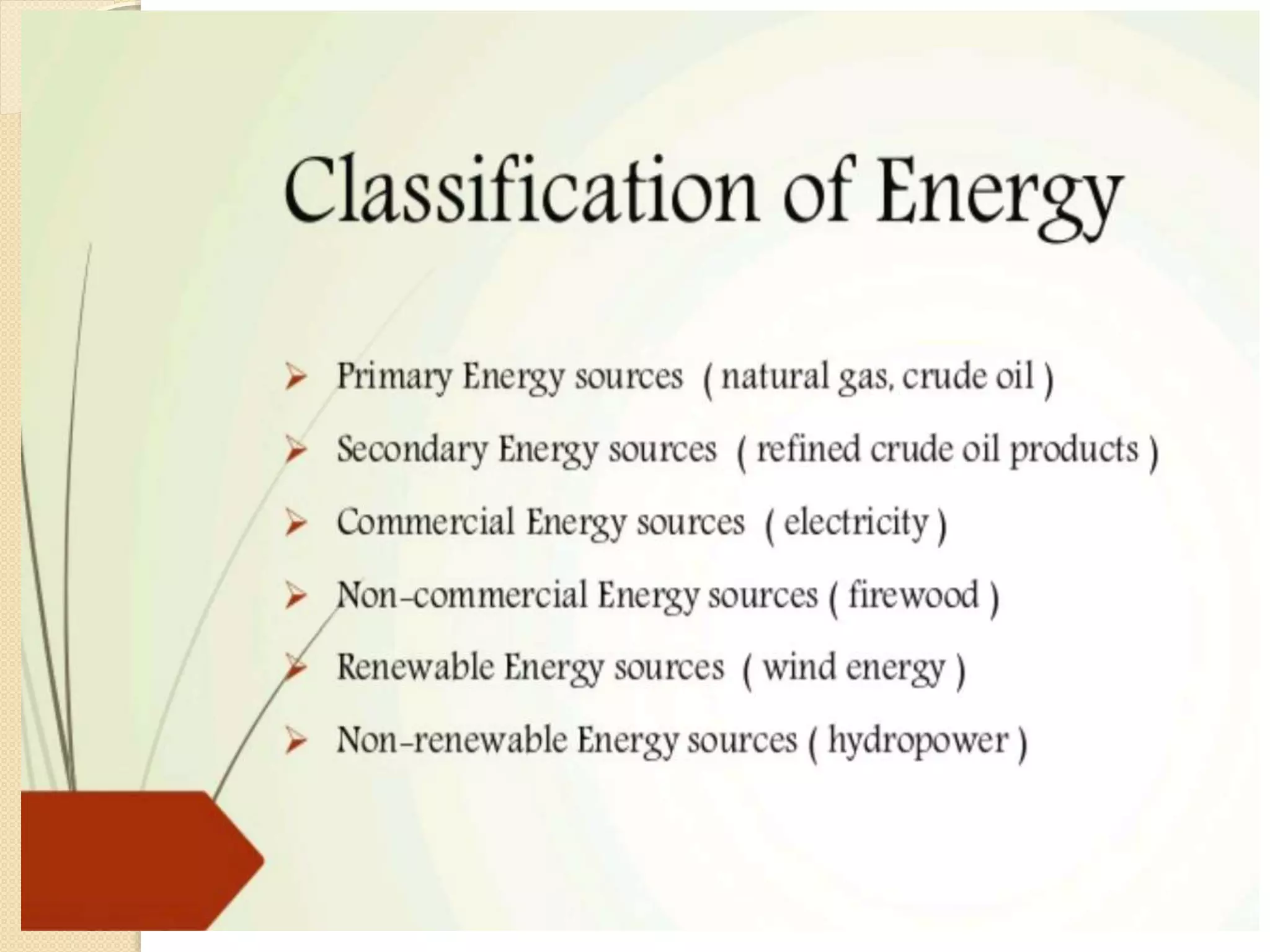
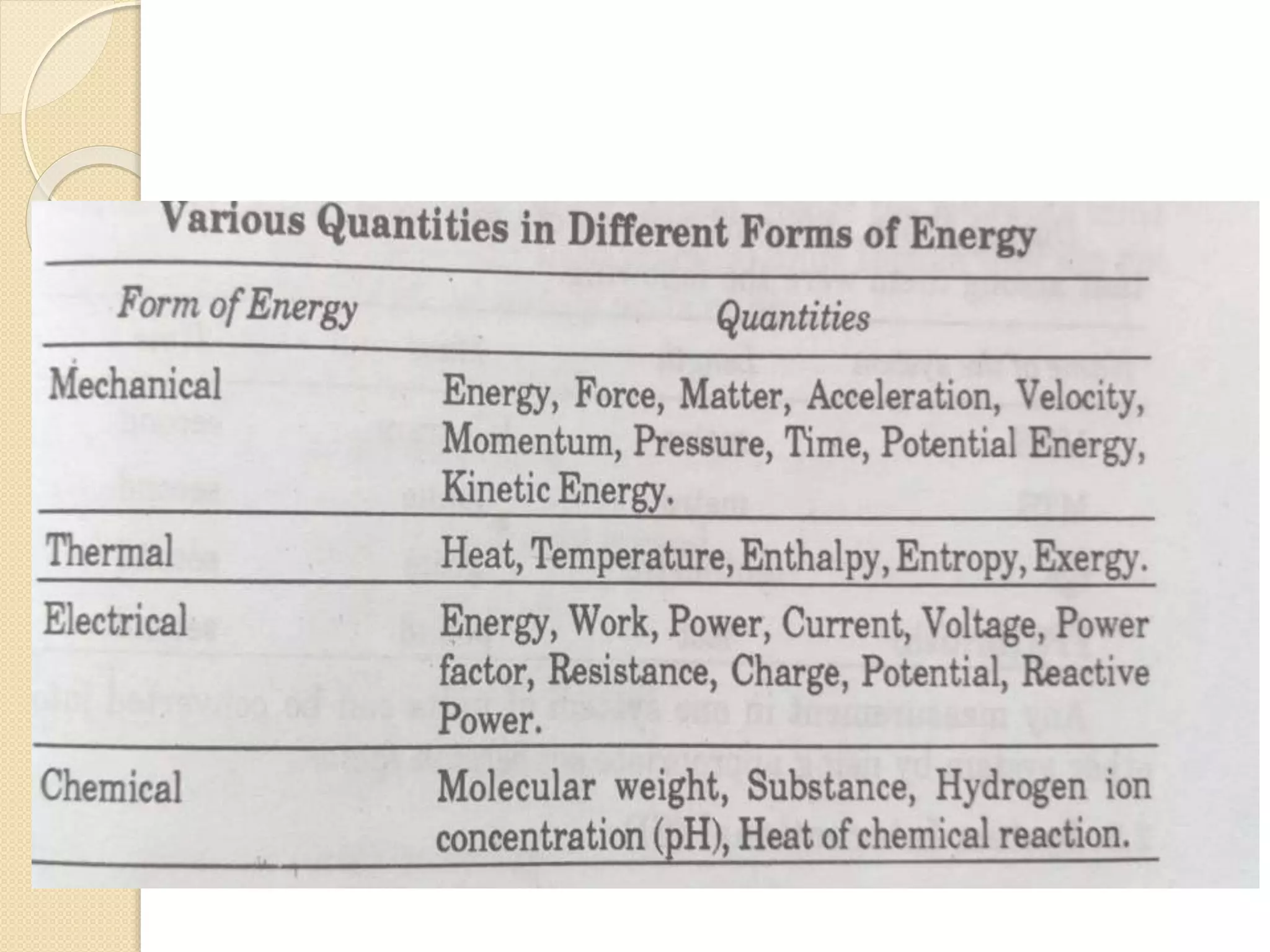
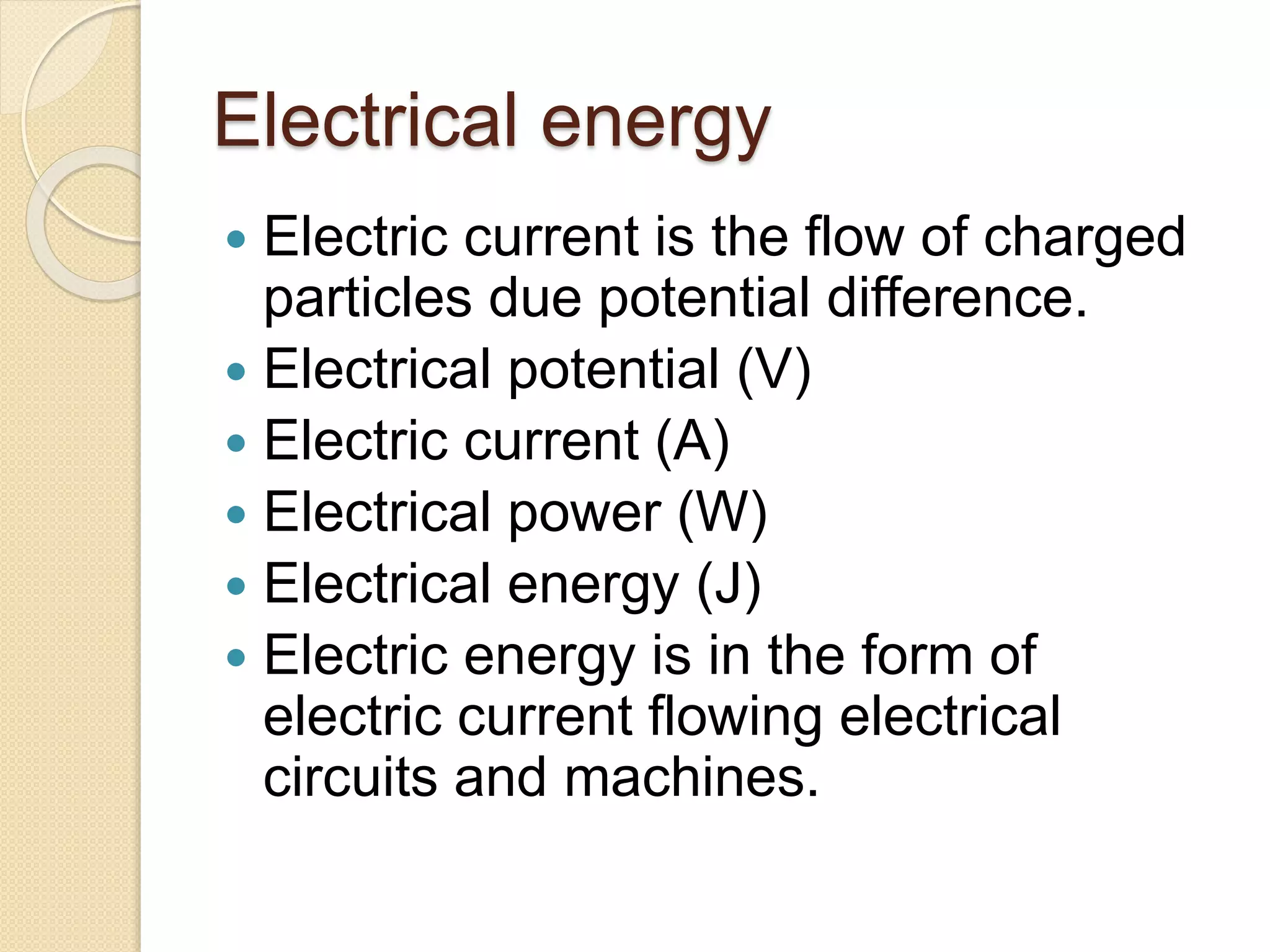
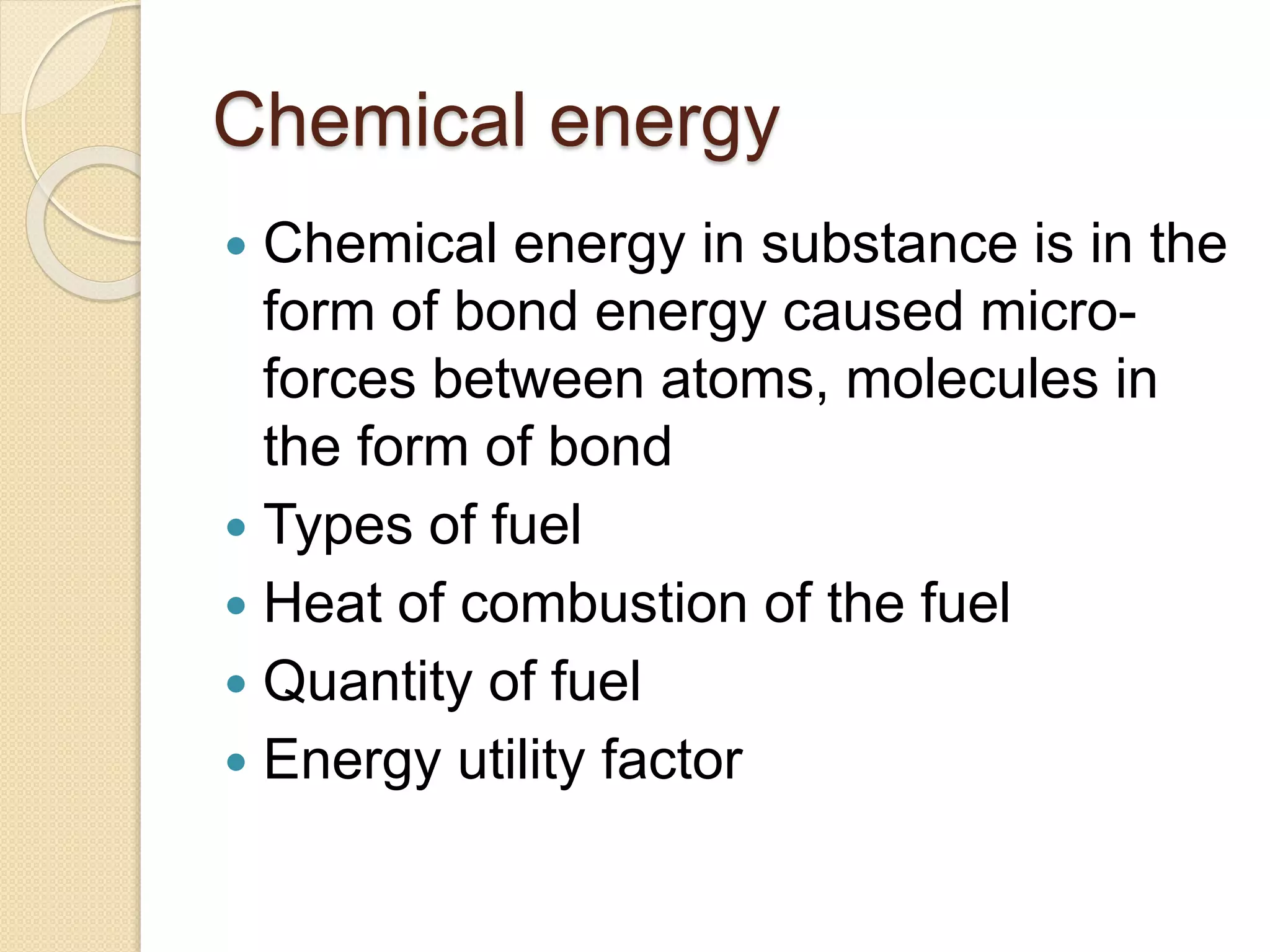
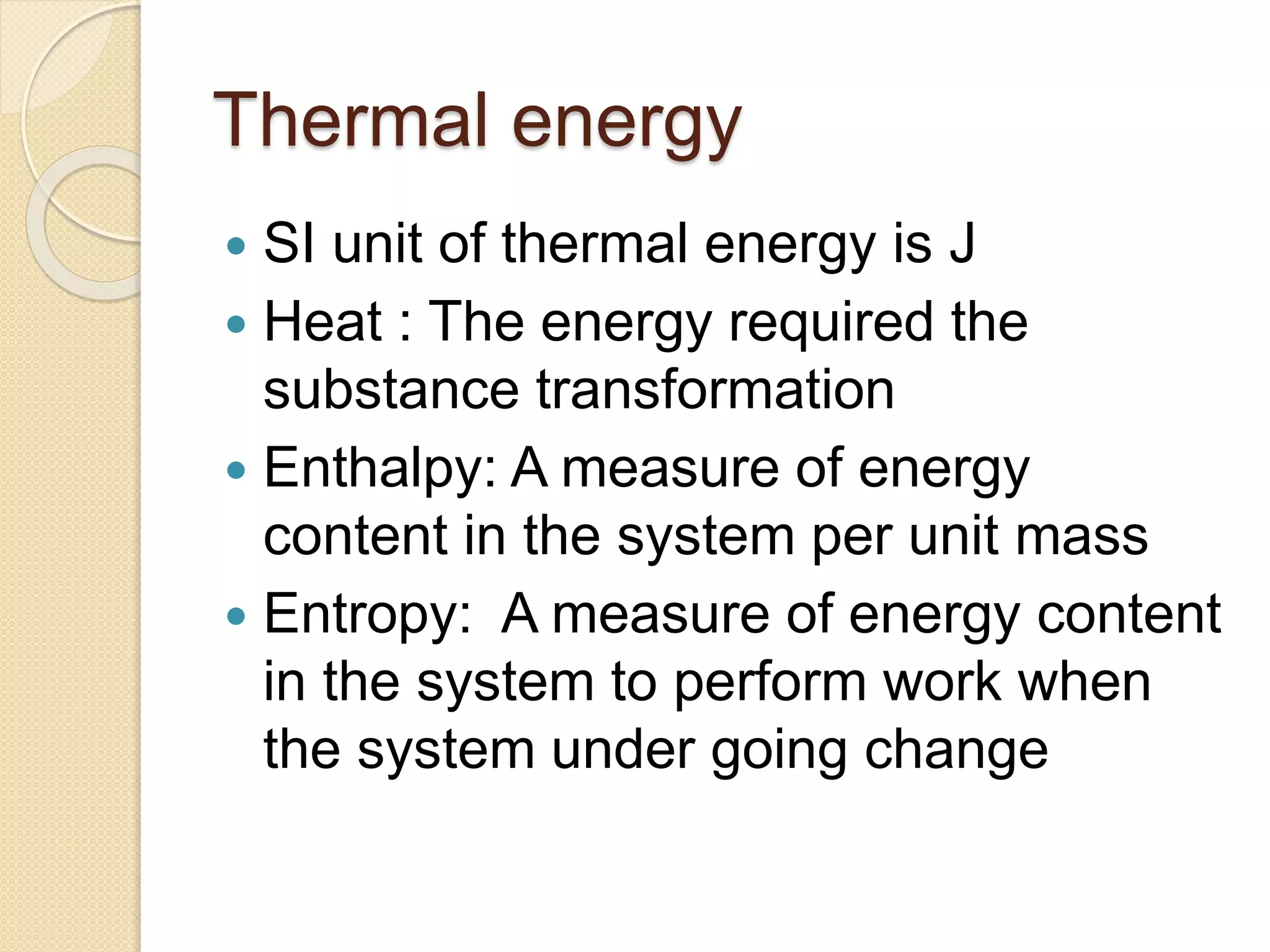
- Descriptions of different types of energy like mechanical, electrical, chemical, and thermal energy.
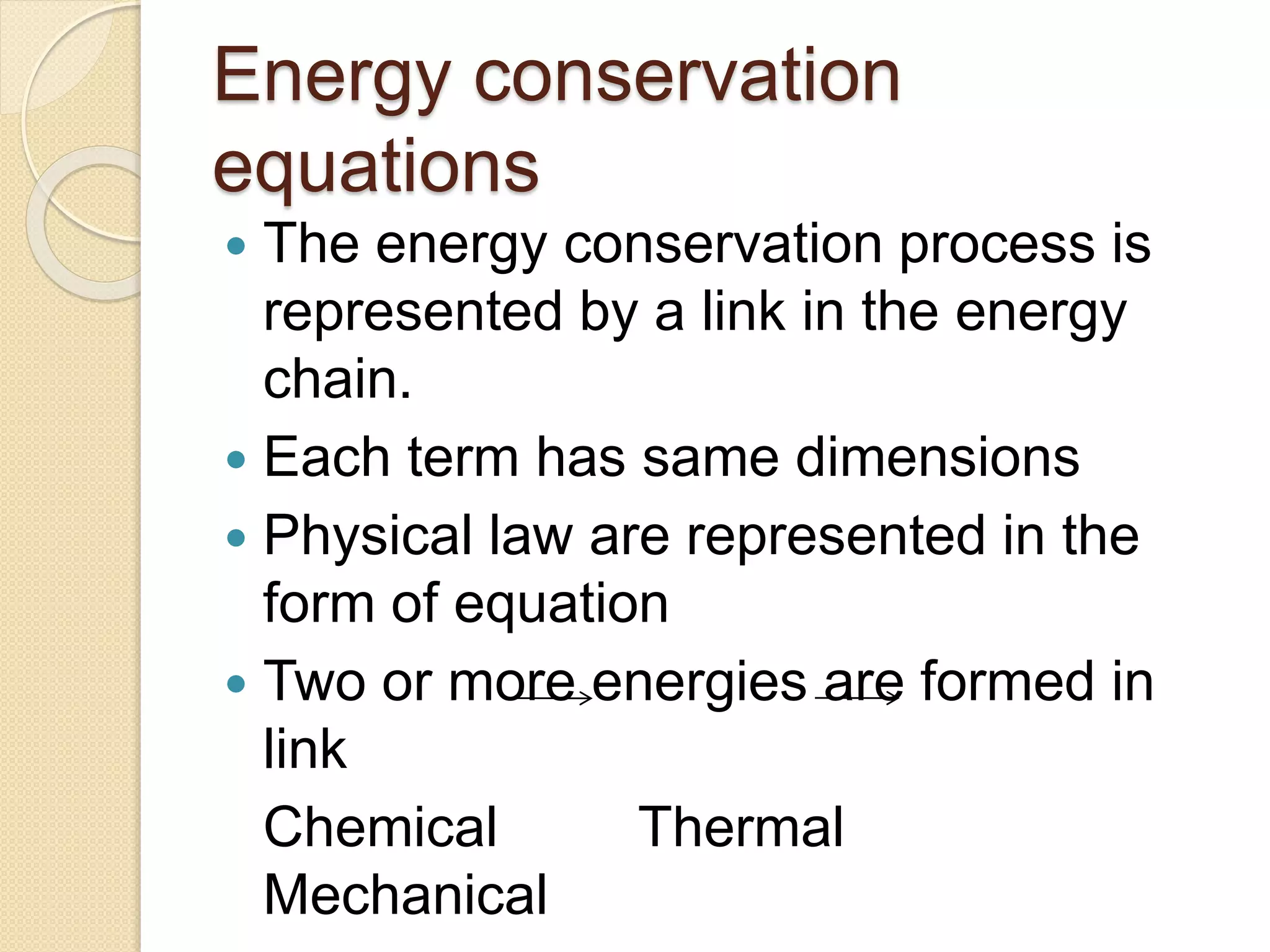
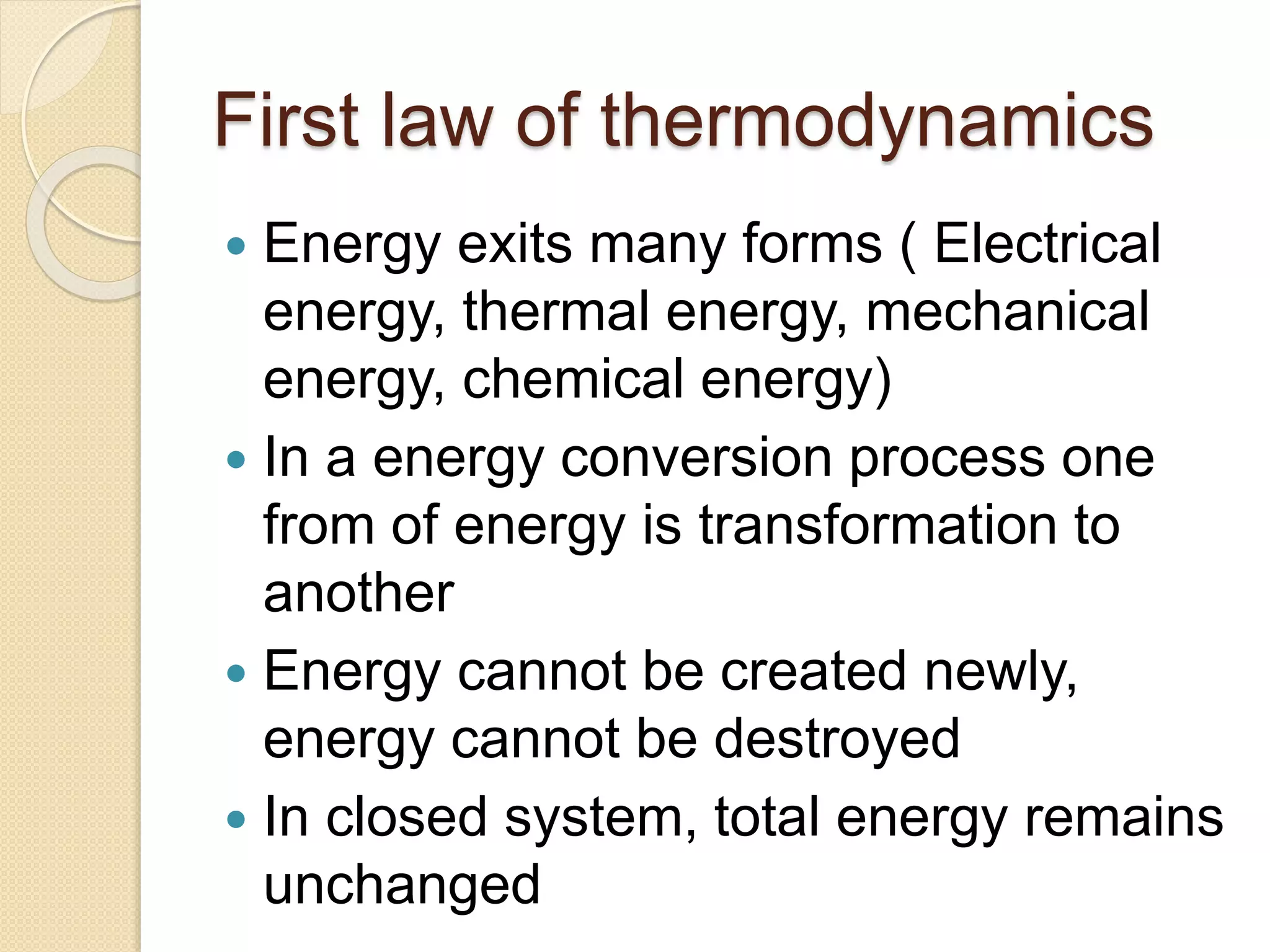
- Summaries of important laws like Newton's laws of motion, laws of thermodynamics, and the law of conservation of energy.
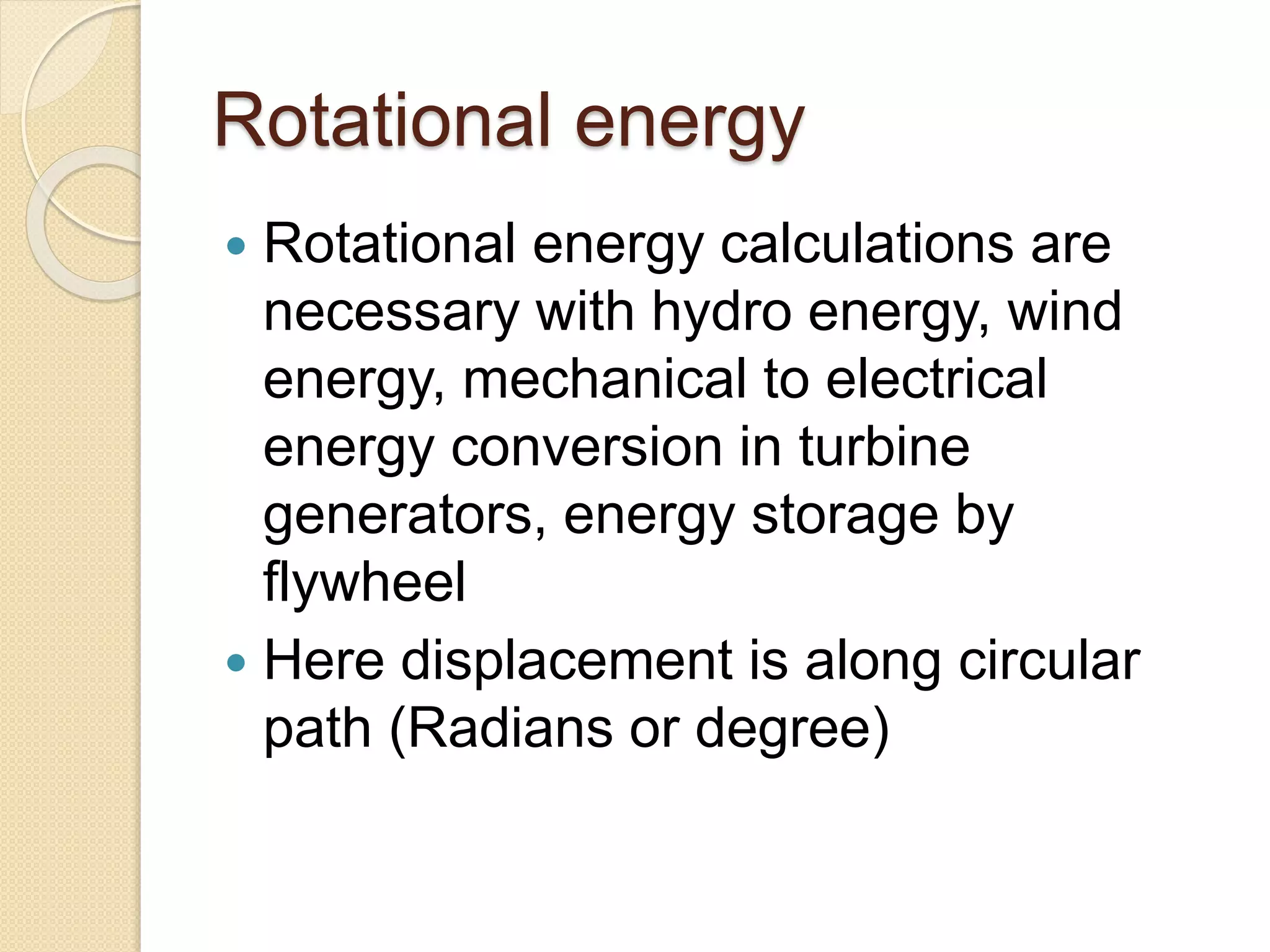
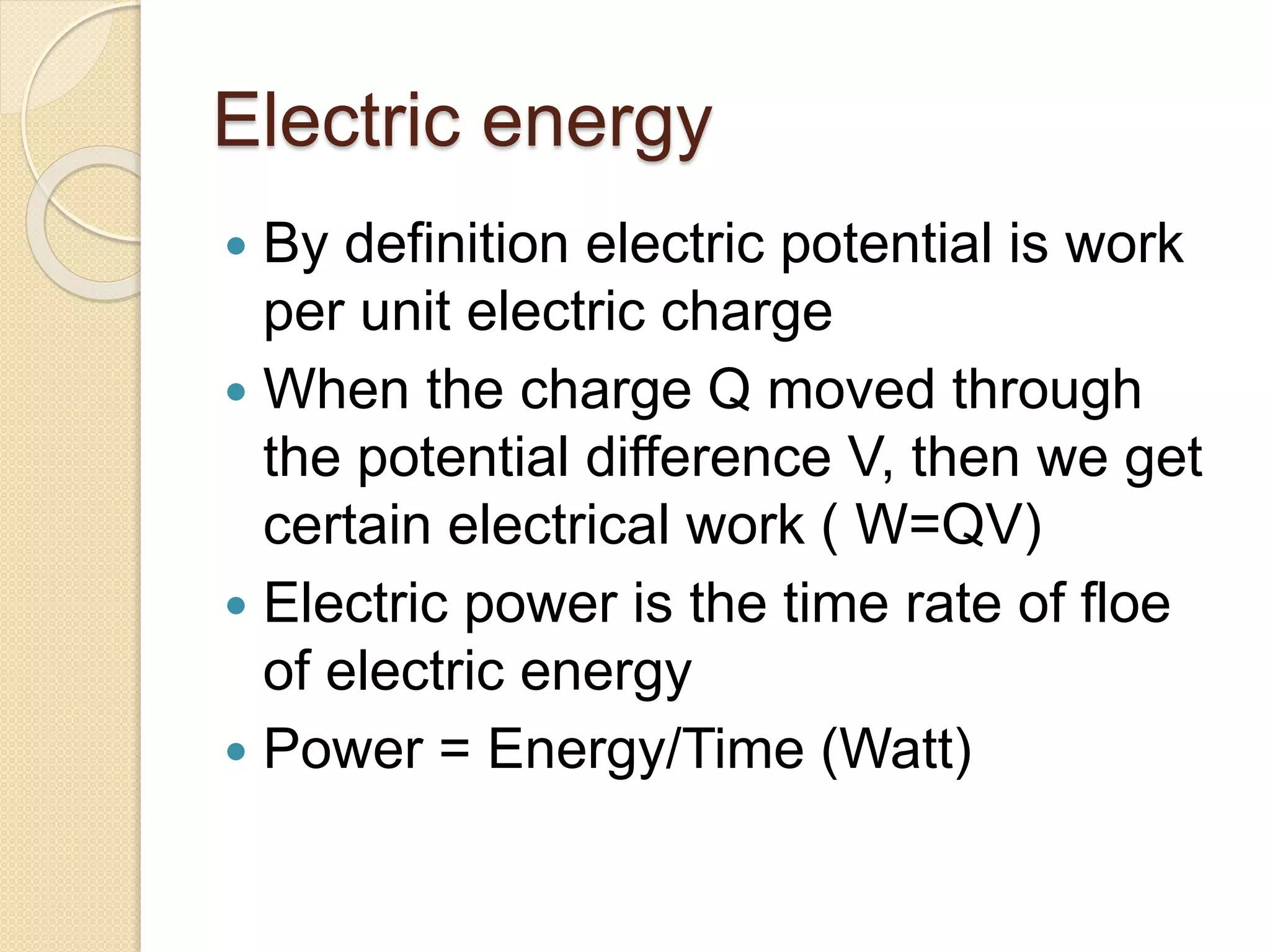
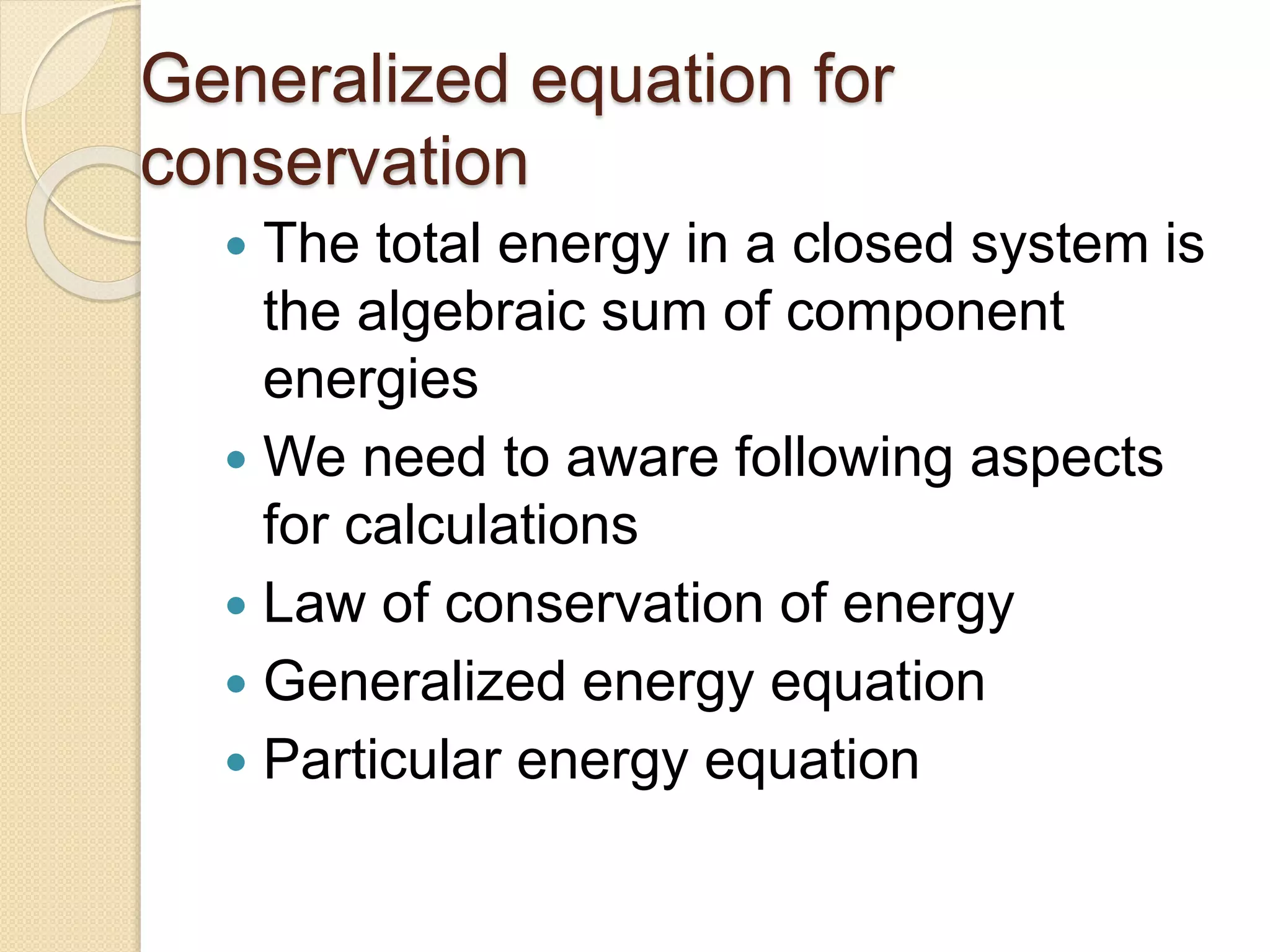
- Examples of dimensional analysis and energy conservation equations.
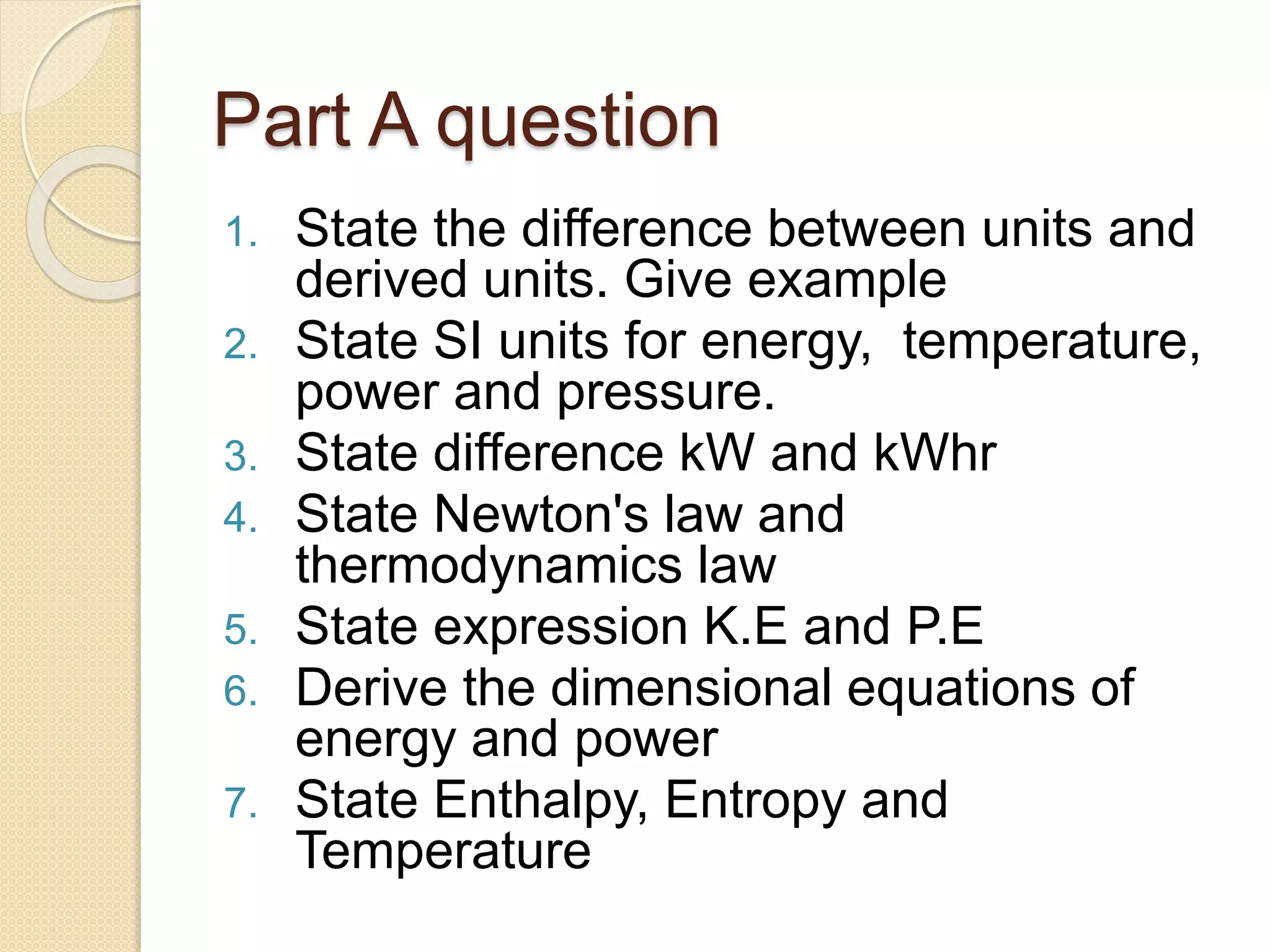
- An outline of exam questions covering these energy concepts.











![Dimensional equations
A quantity can be represented by a
dimensional equation without
reference to units
Dimensions of force f are derived as
follows
F=mass x acceleration
◦ = mass x (change in velocity/time)
◦ [F]=[M] [L]/[T2]
◦](https://image.slidesharecdn.com/l3-unit1energyunitsofenergyconversionfactorsclassification-200901110656/75/L3-unit-1-energy-units-of-energy-conversion-factors-classification-25-2048.jpg)