The document discusses various topics related to biomass energy including:
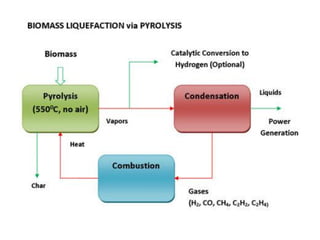
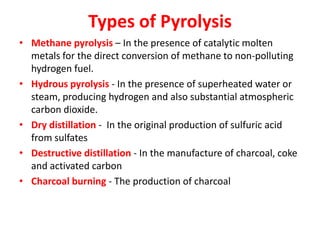
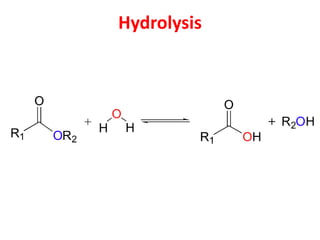
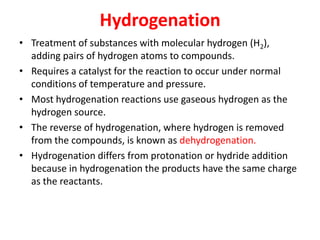
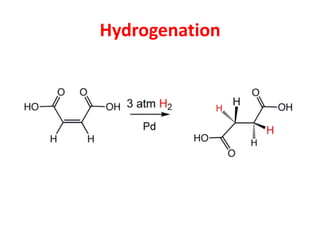
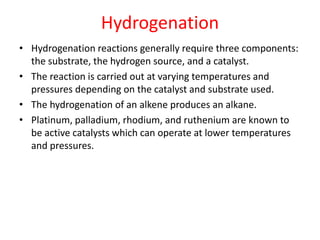
- Types of biomass gasification such as pyrolysis, hydrolysis, hydrogenation, and gasification. Pyrolysis involves thermal decomposition of biomass in an inert atmosphere. Hydrolysis uses water to break chemical bonds. Hydrogenation treats substances with hydrogen gas.
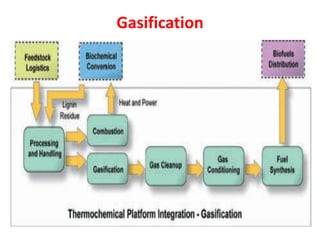
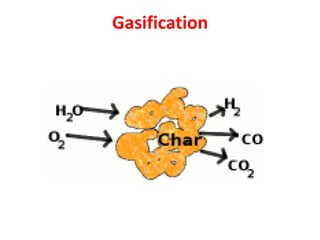
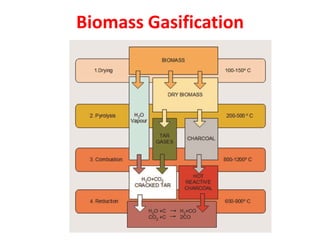
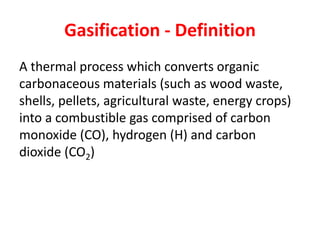
- Gasification is a process that converts biomass into syngas (carbon monoxide and hydrogen) using heat in the absence of oxygen.
- Biodiesel production involves transesterification of vegetable oils or animal fats with methanol in the presence of a catalyst to produce biodiesel and glycerin.
- Biomass can be used to generate
















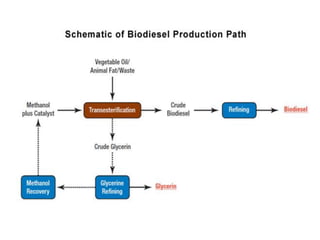
![Biodiesel Production
• Produced from vegetable oils, yellow grease, used cooking
oils, or animal fats
• Produced by transesterification—a process that converts fats
and oils into biodiesel and glycerin (a byproduct)
• Approximately 100 pounds of oil or fat are reacted with 10
pounds of a short-chain alcohol (usually methanol) in the
presence of a catalyst (usually sodium hydroxide [NaOH] or
potassium hydroxide [KOH])
• 100 pounds of biodiesel and 10 pounds of glycerin (or
glycerol) will be produced.
• Glycerin, a co-product, is a sugar commonly used in the
manufacture of pharmaceuticals and cosmetics.](https://image.slidesharecdn.com/och752energytechnologyunit4-201103122532/85/Och-752-energy-technology-unit-4-46-320.jpg)
