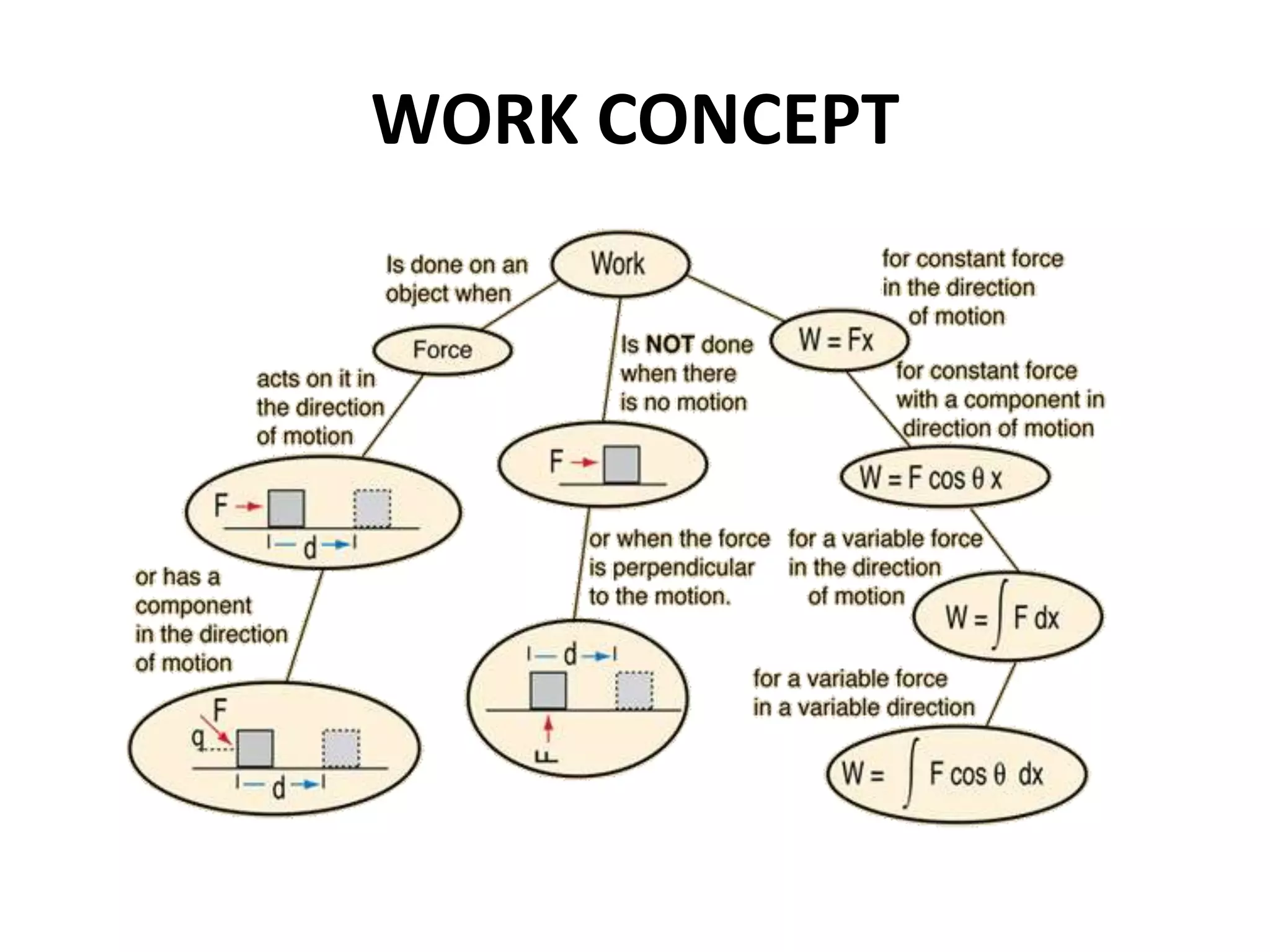
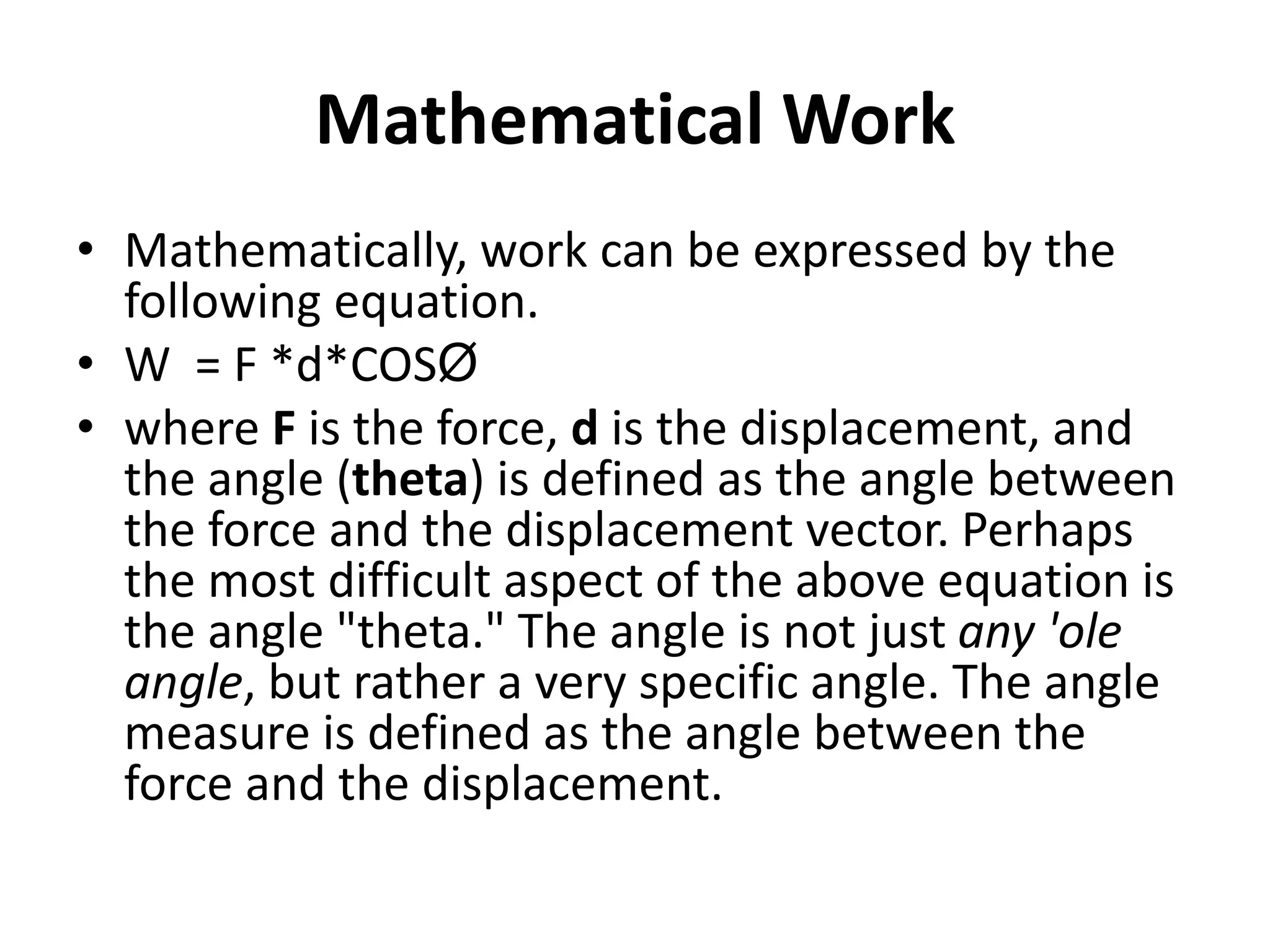
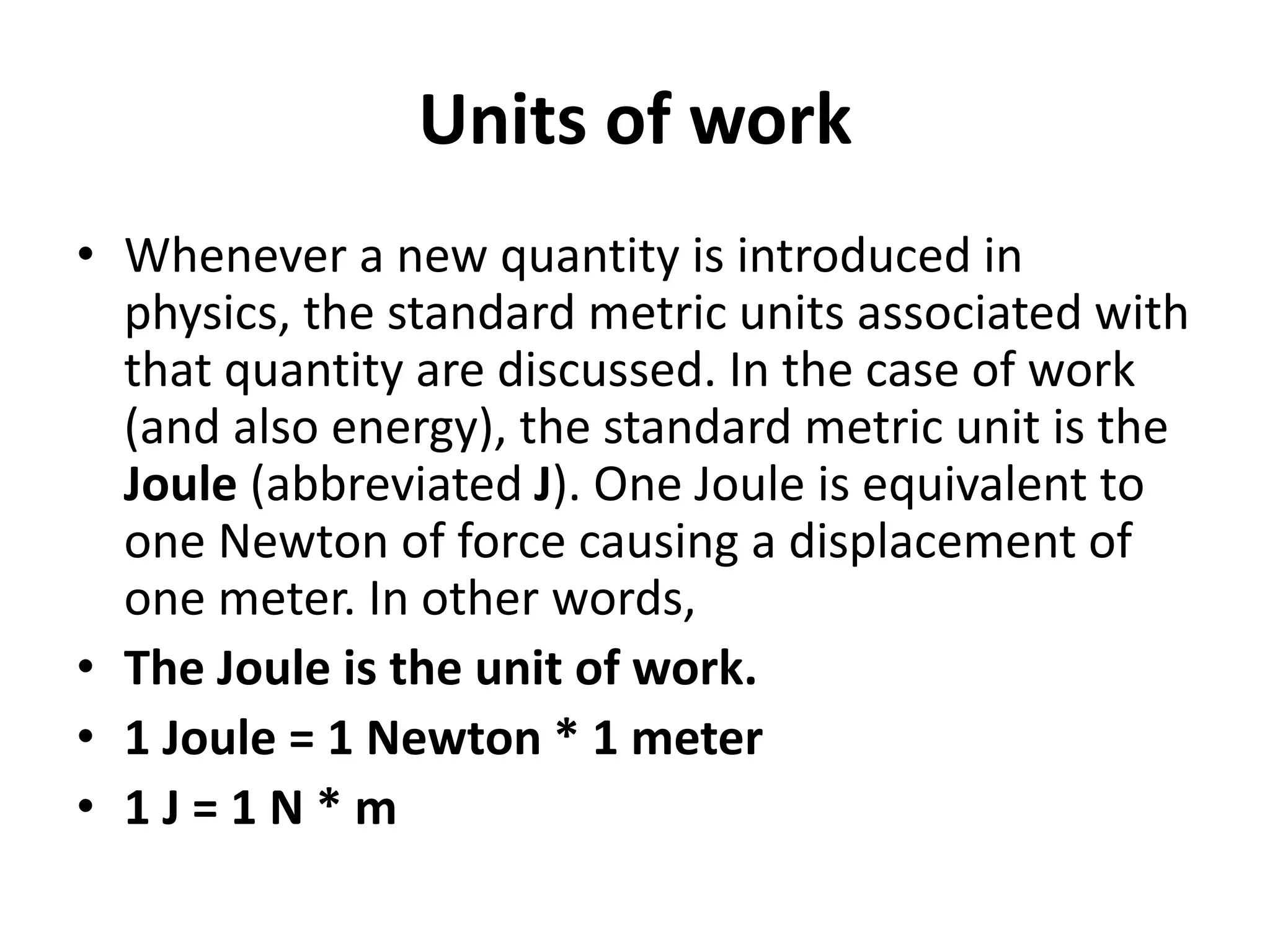
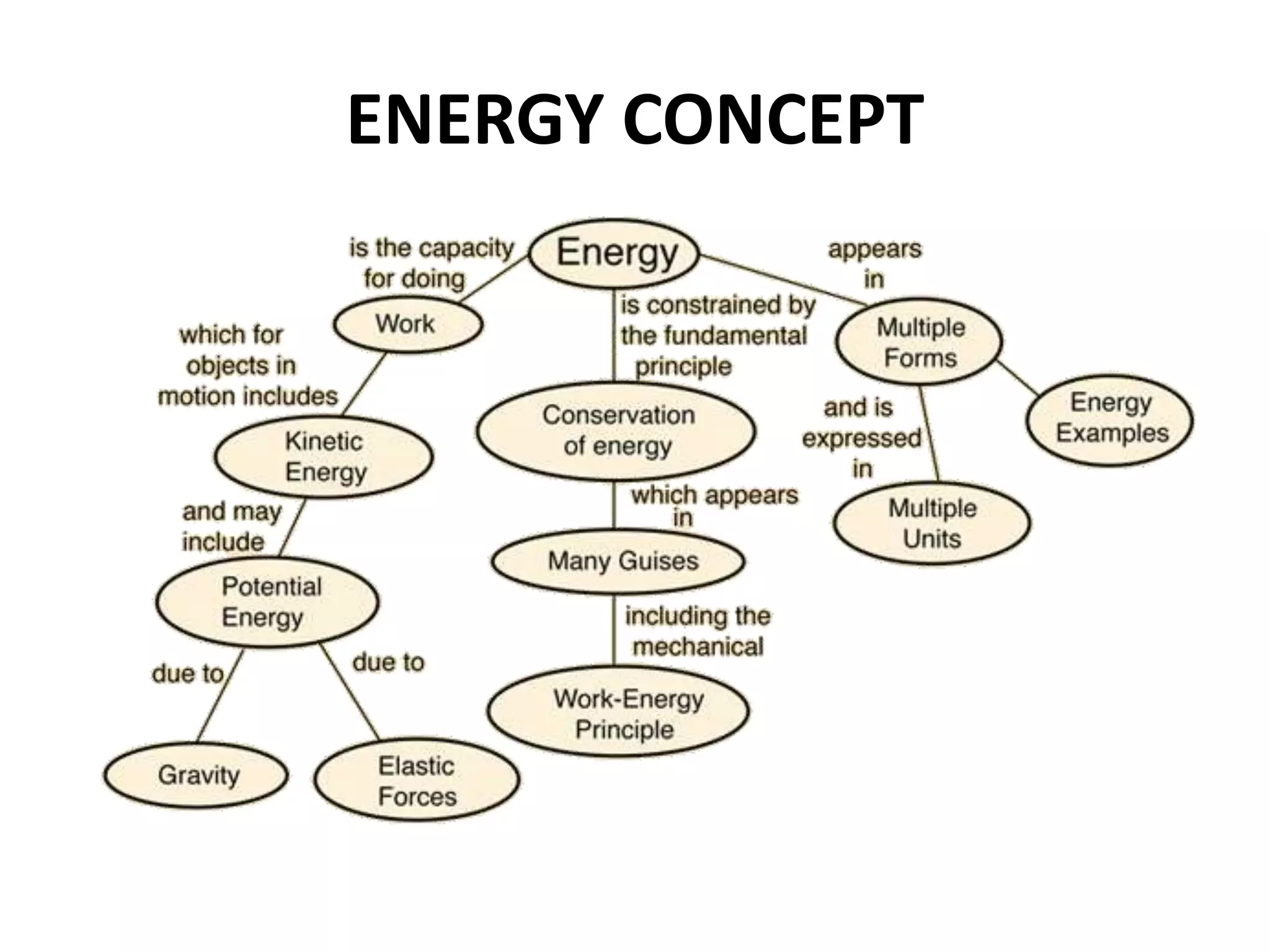
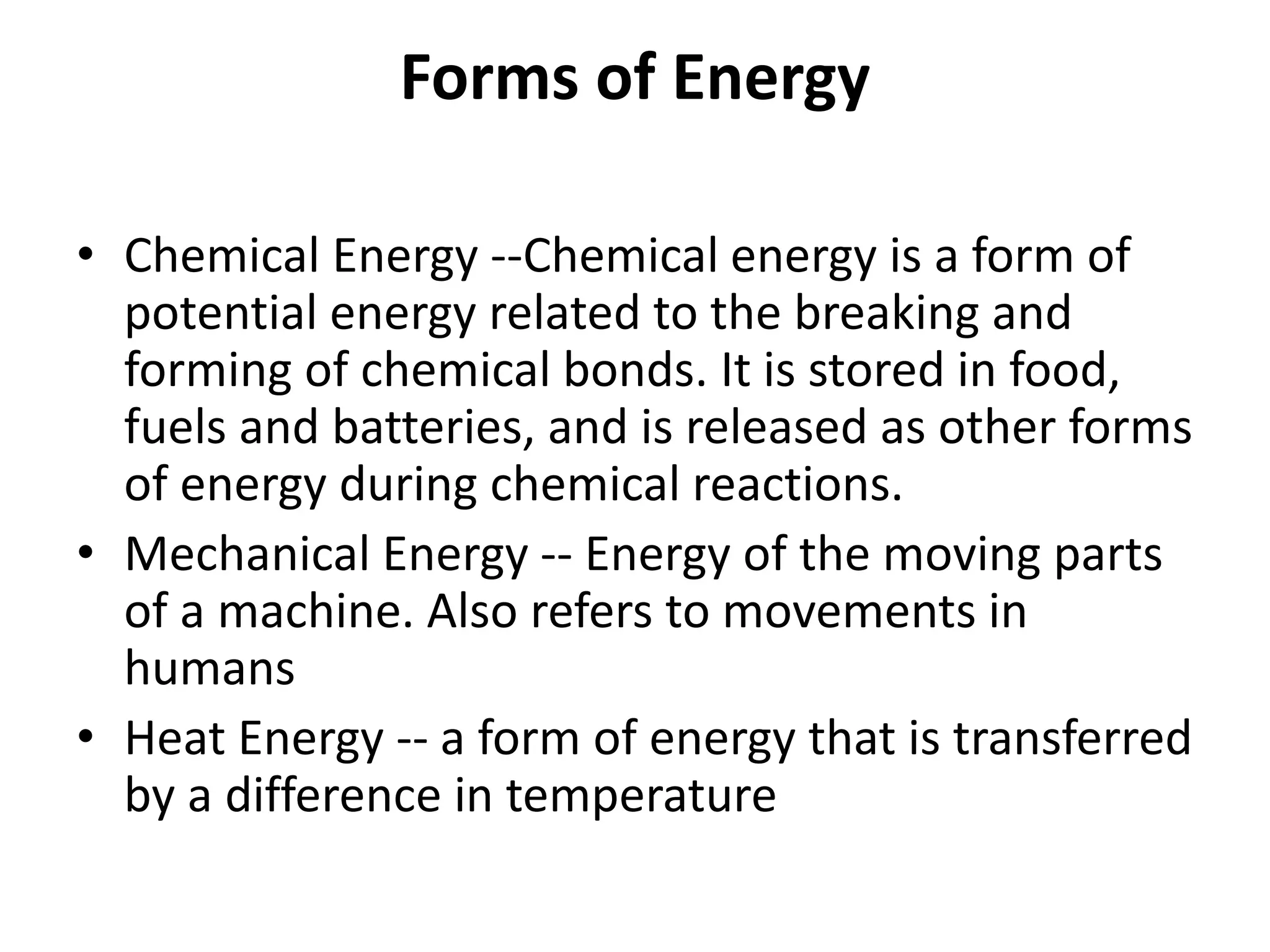
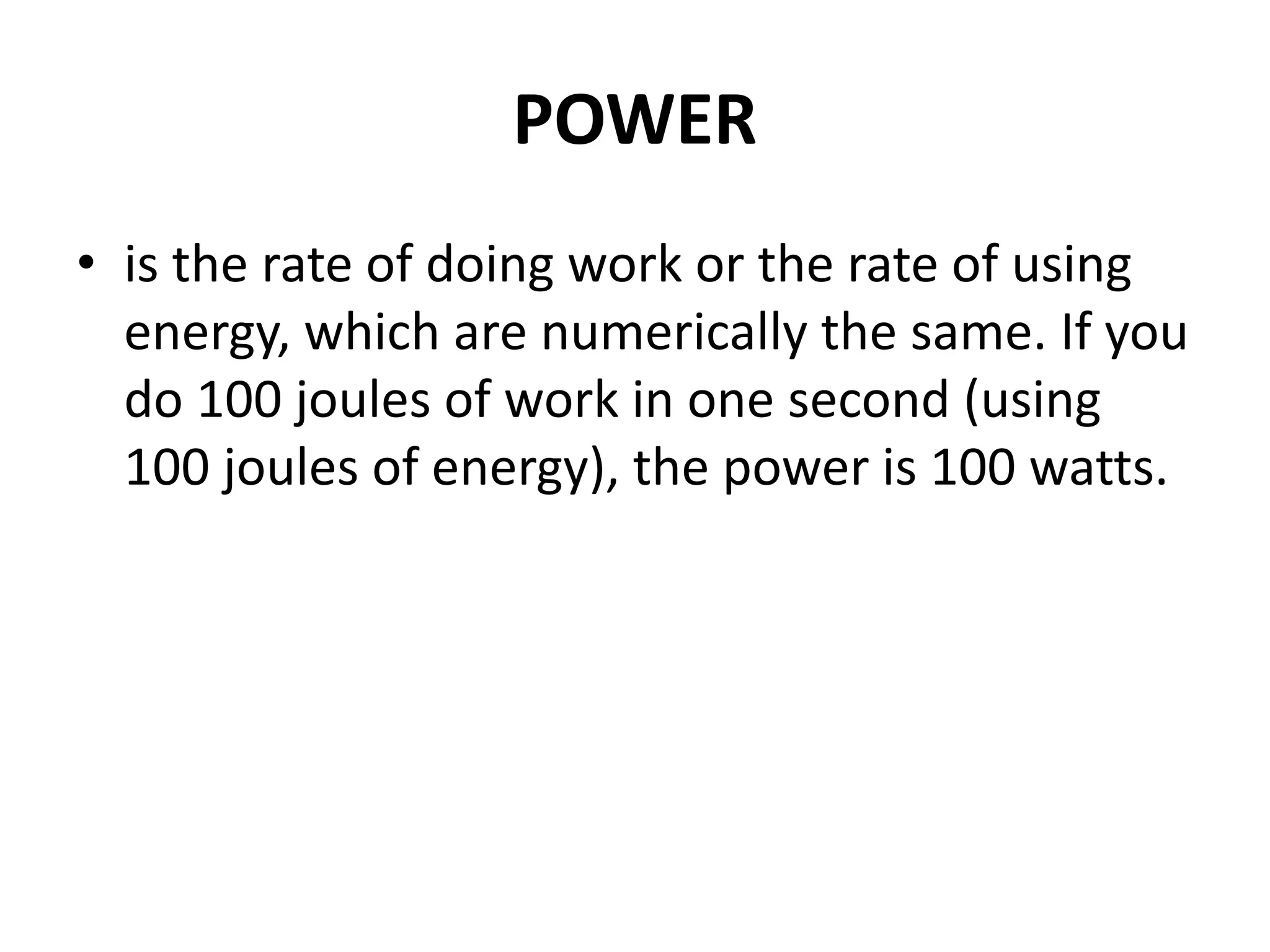
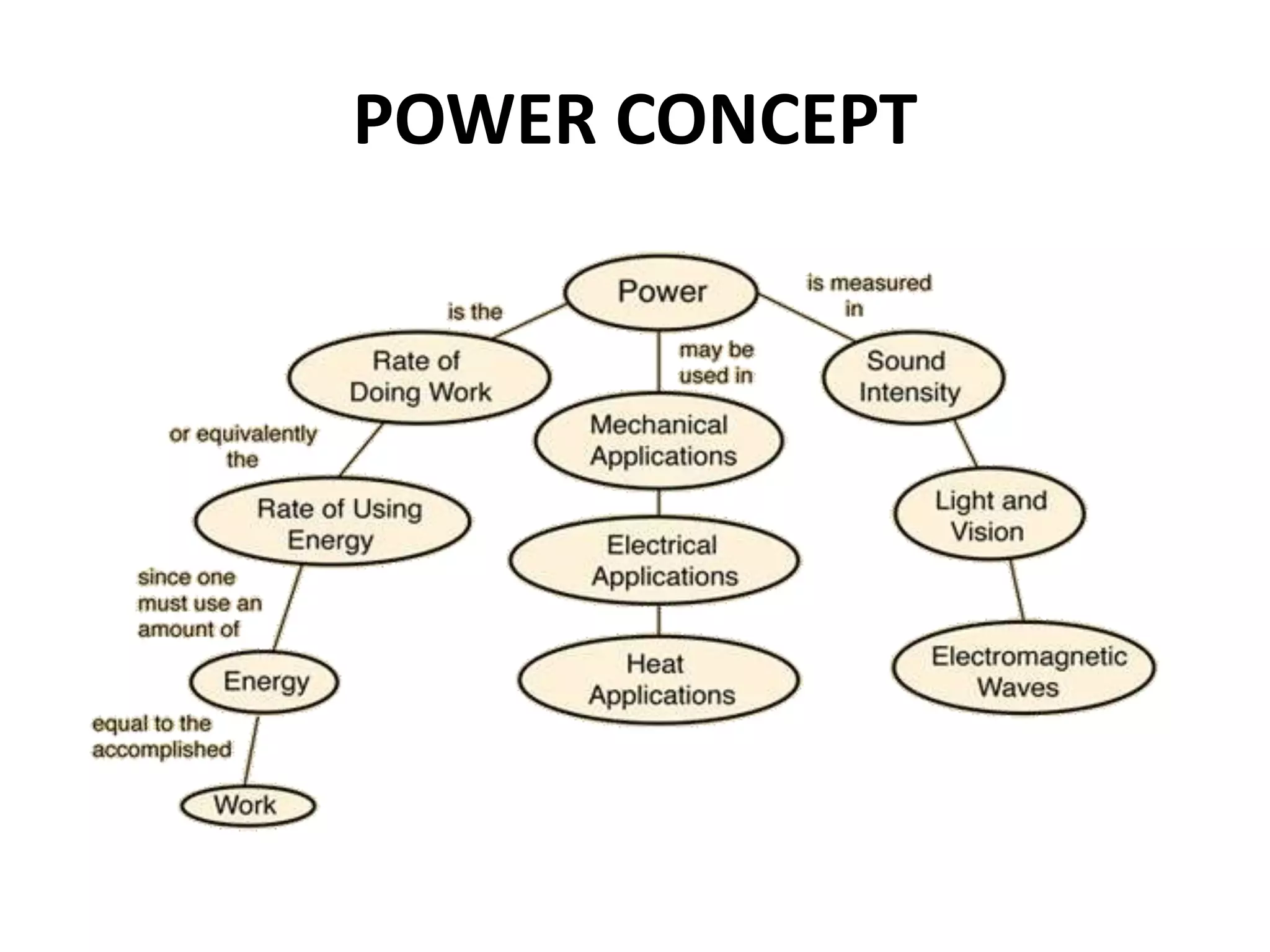
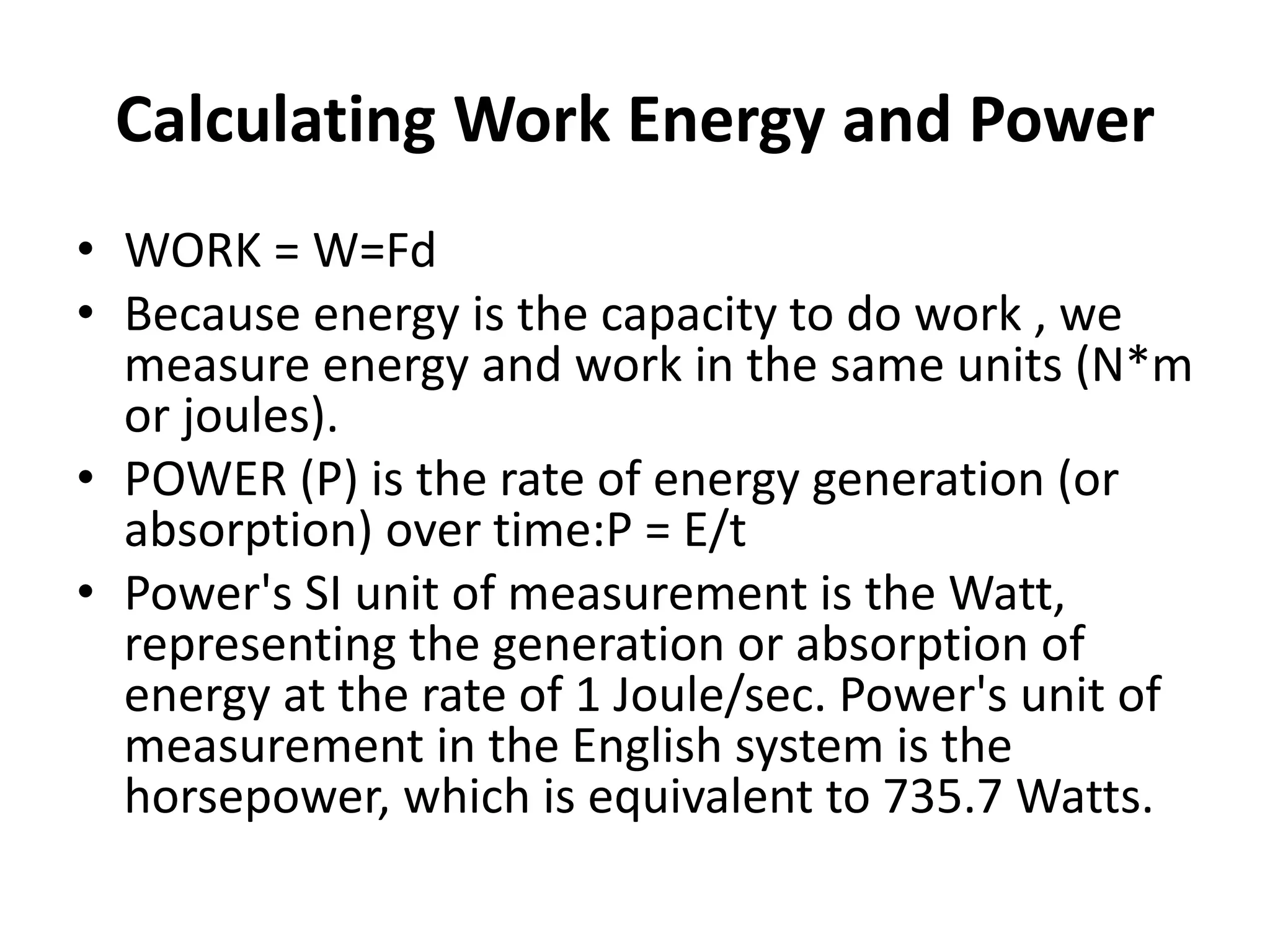
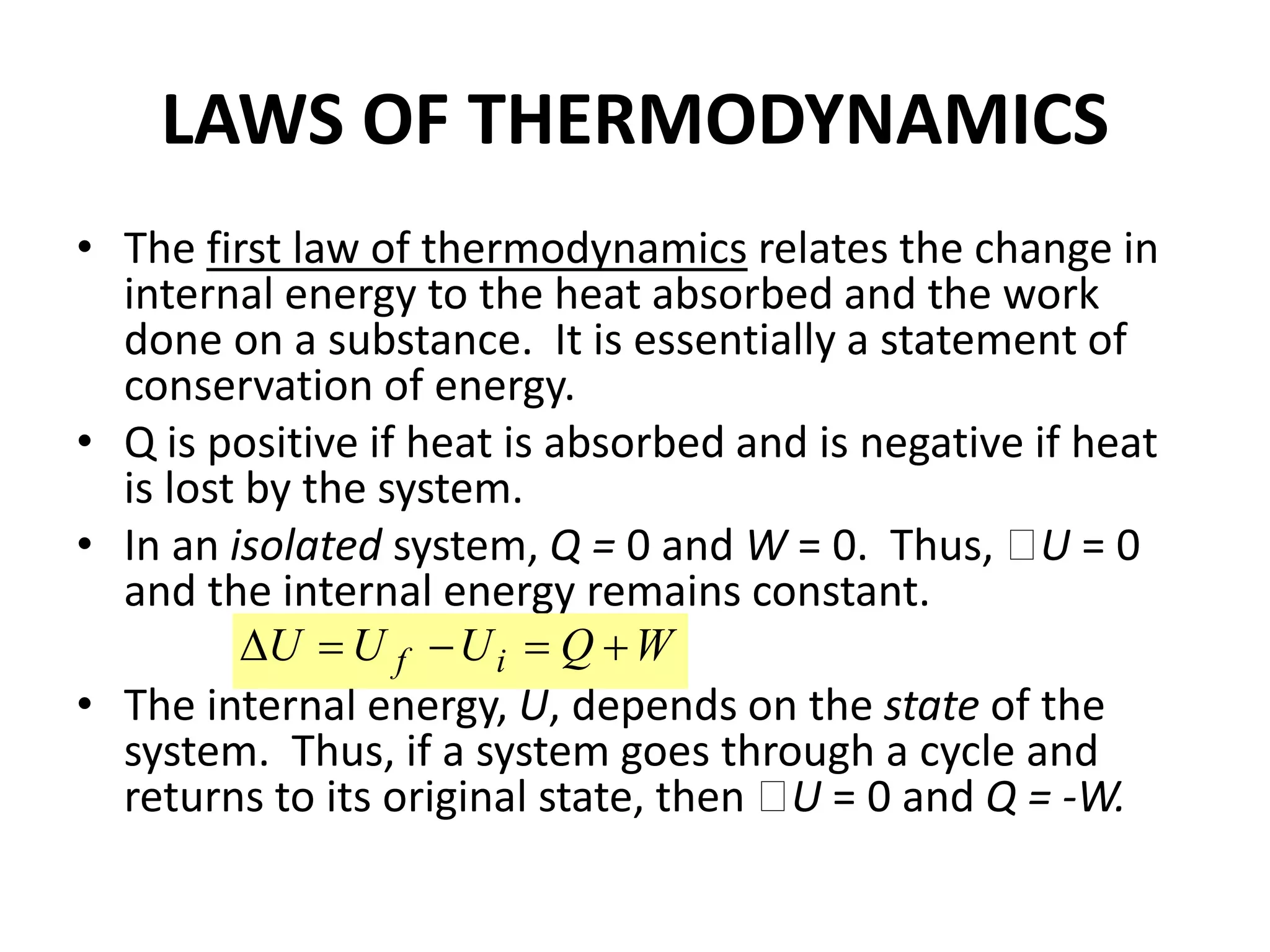
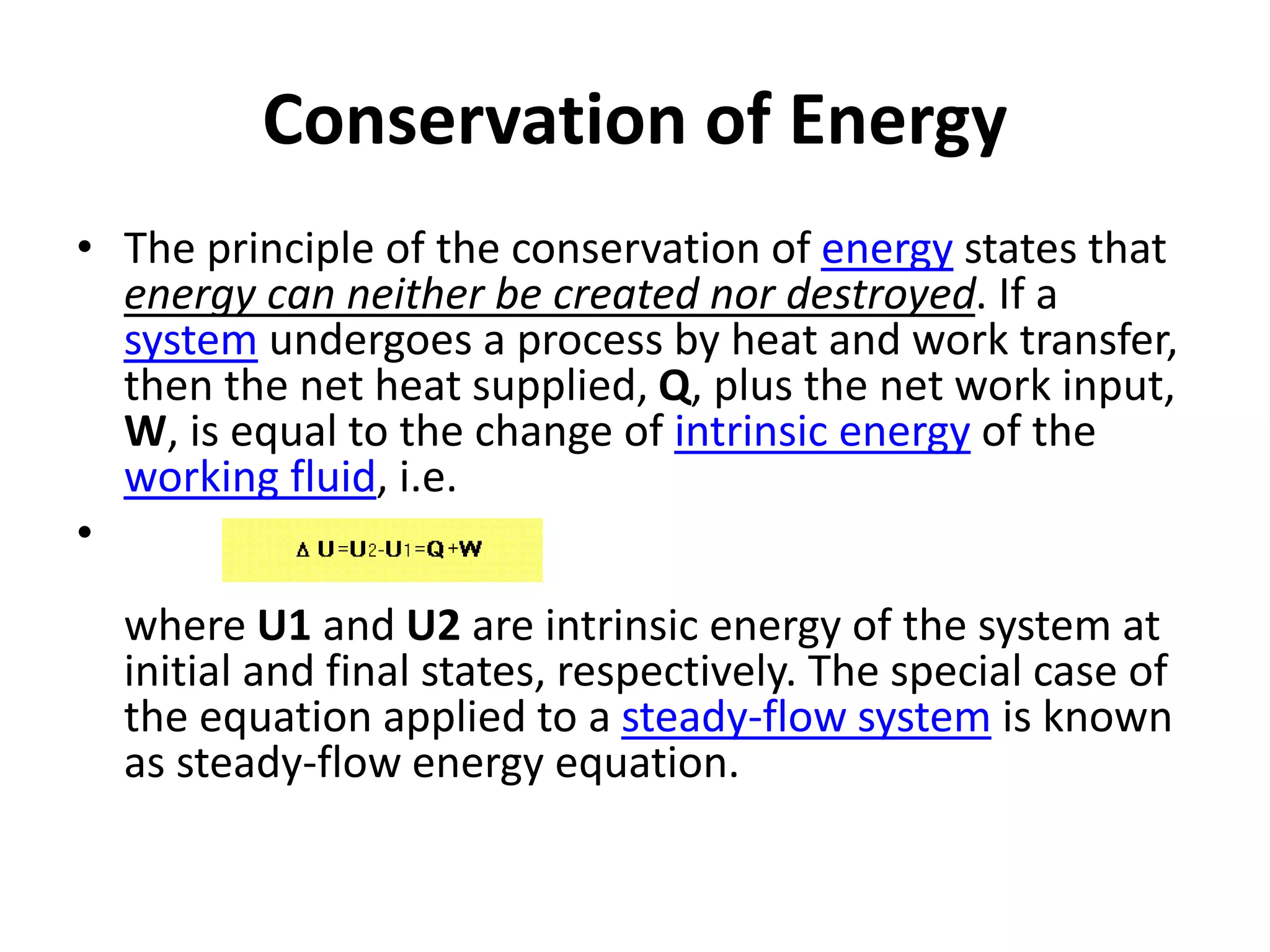
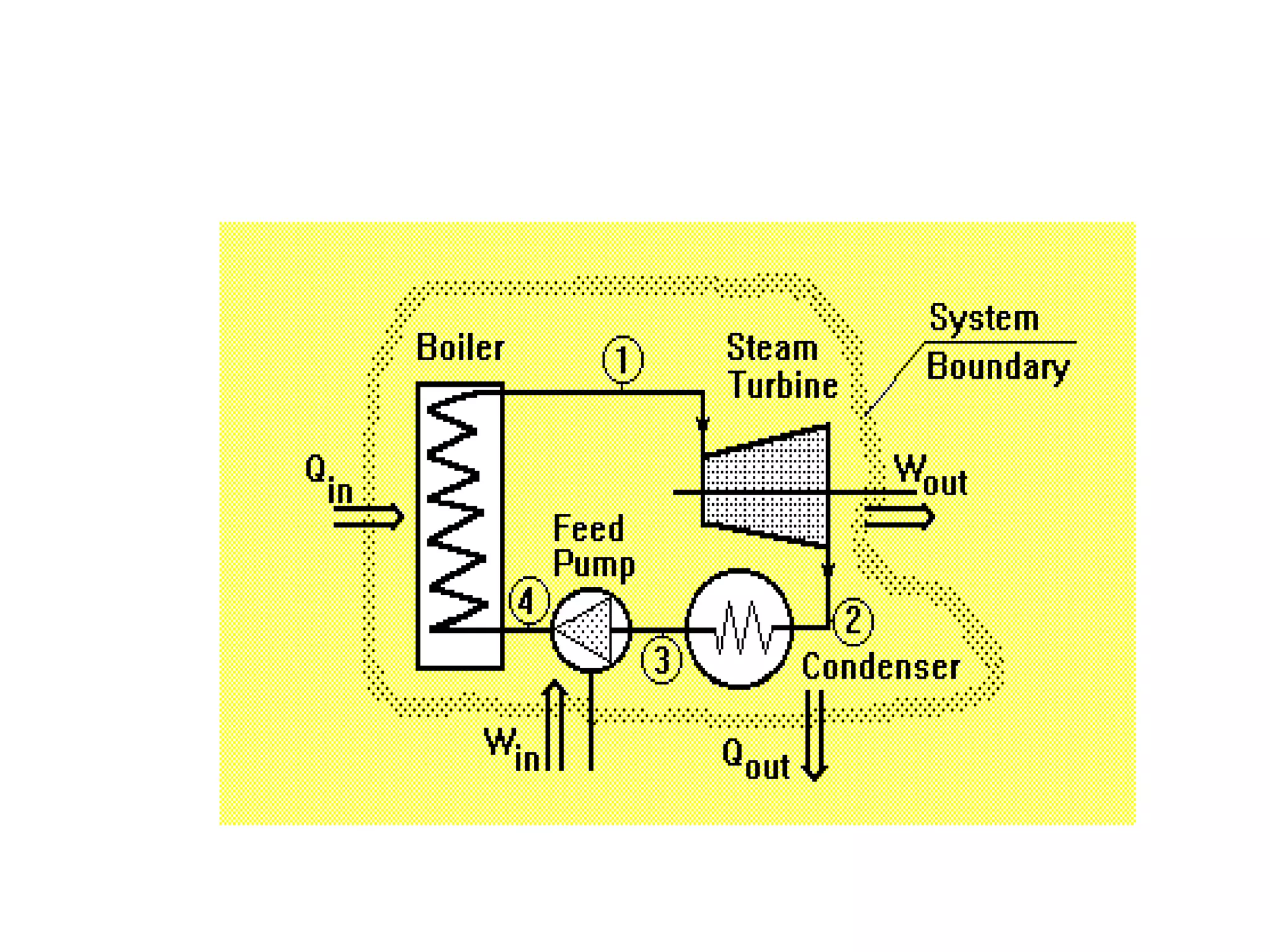
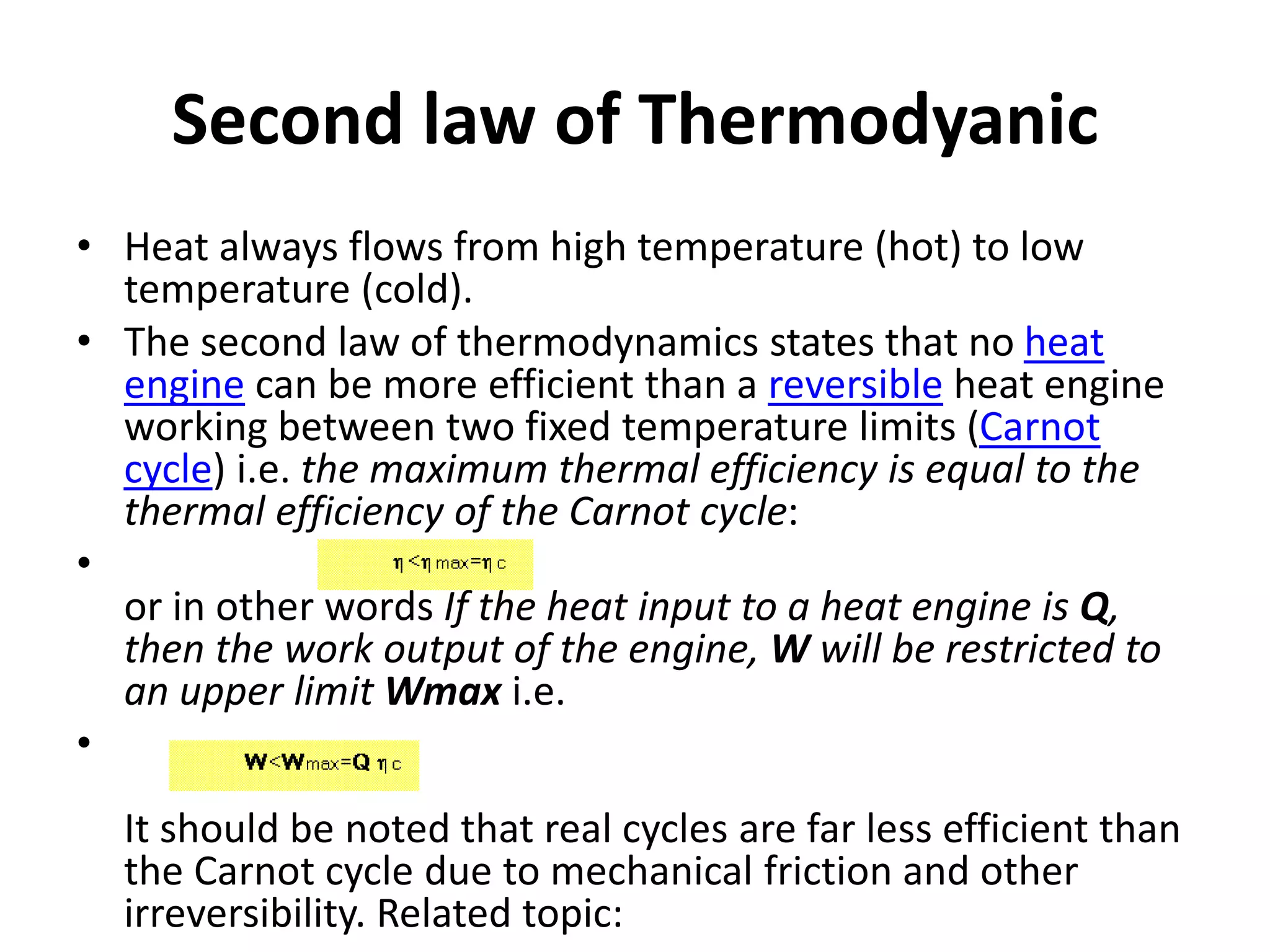
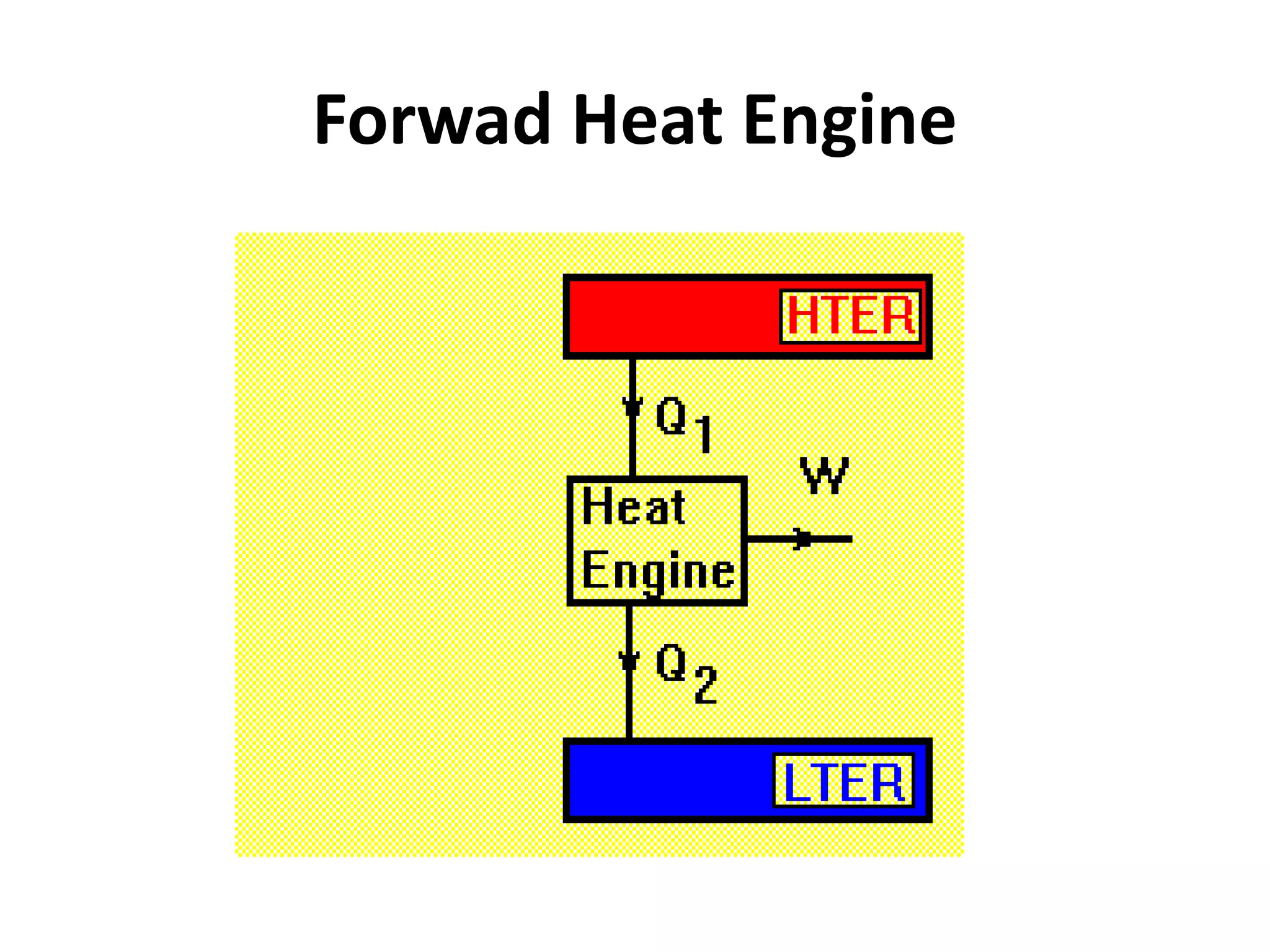
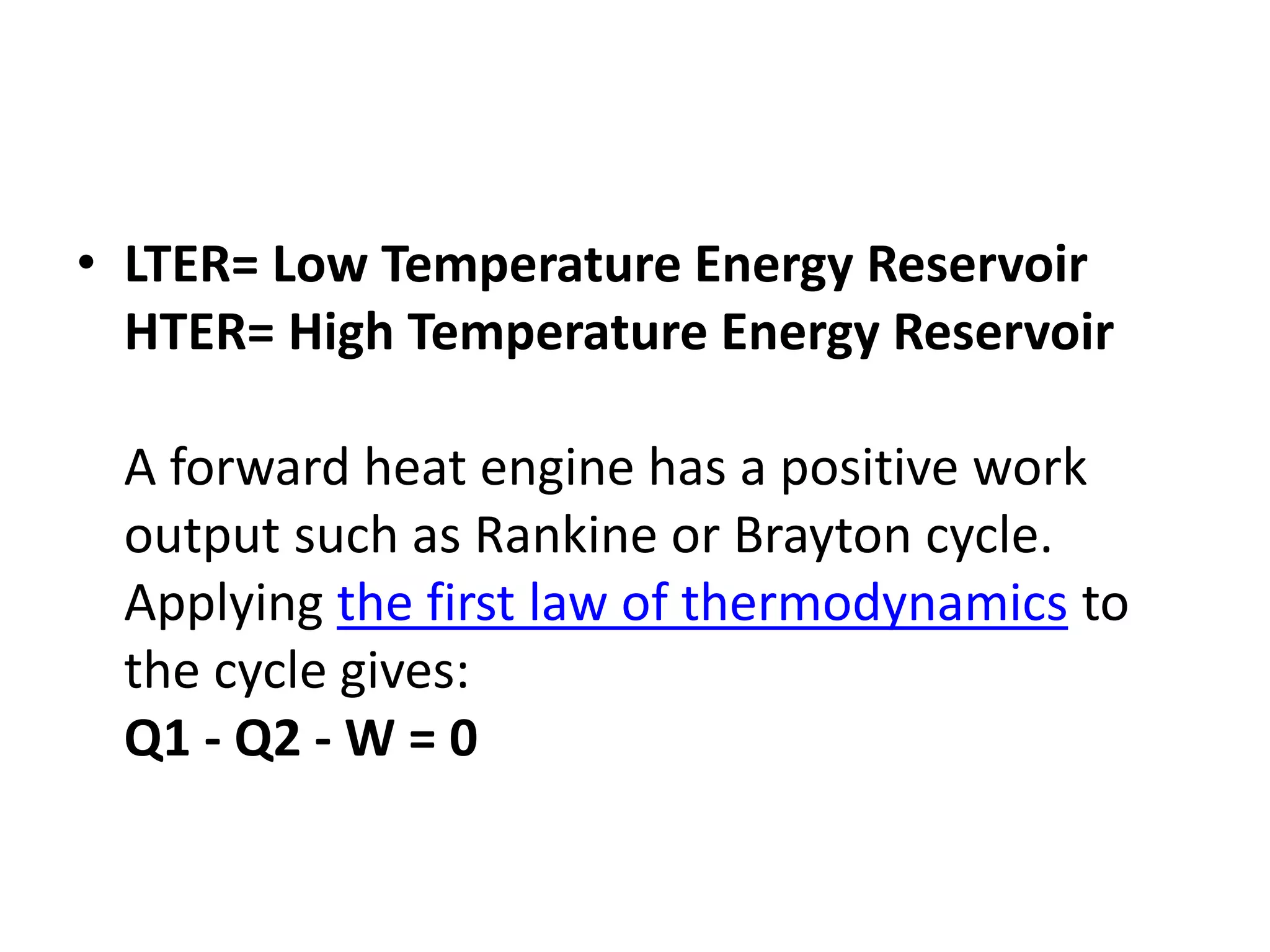
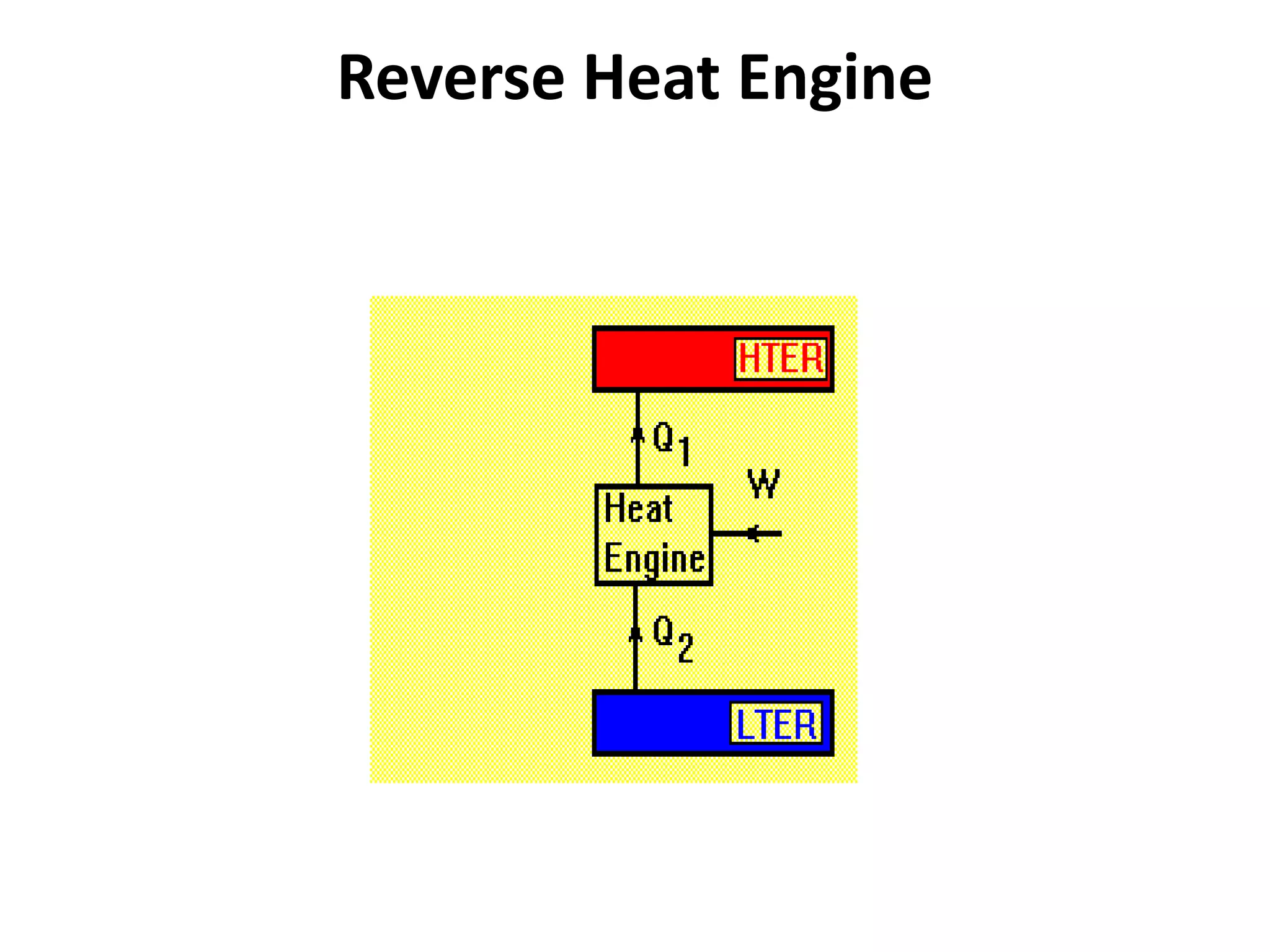
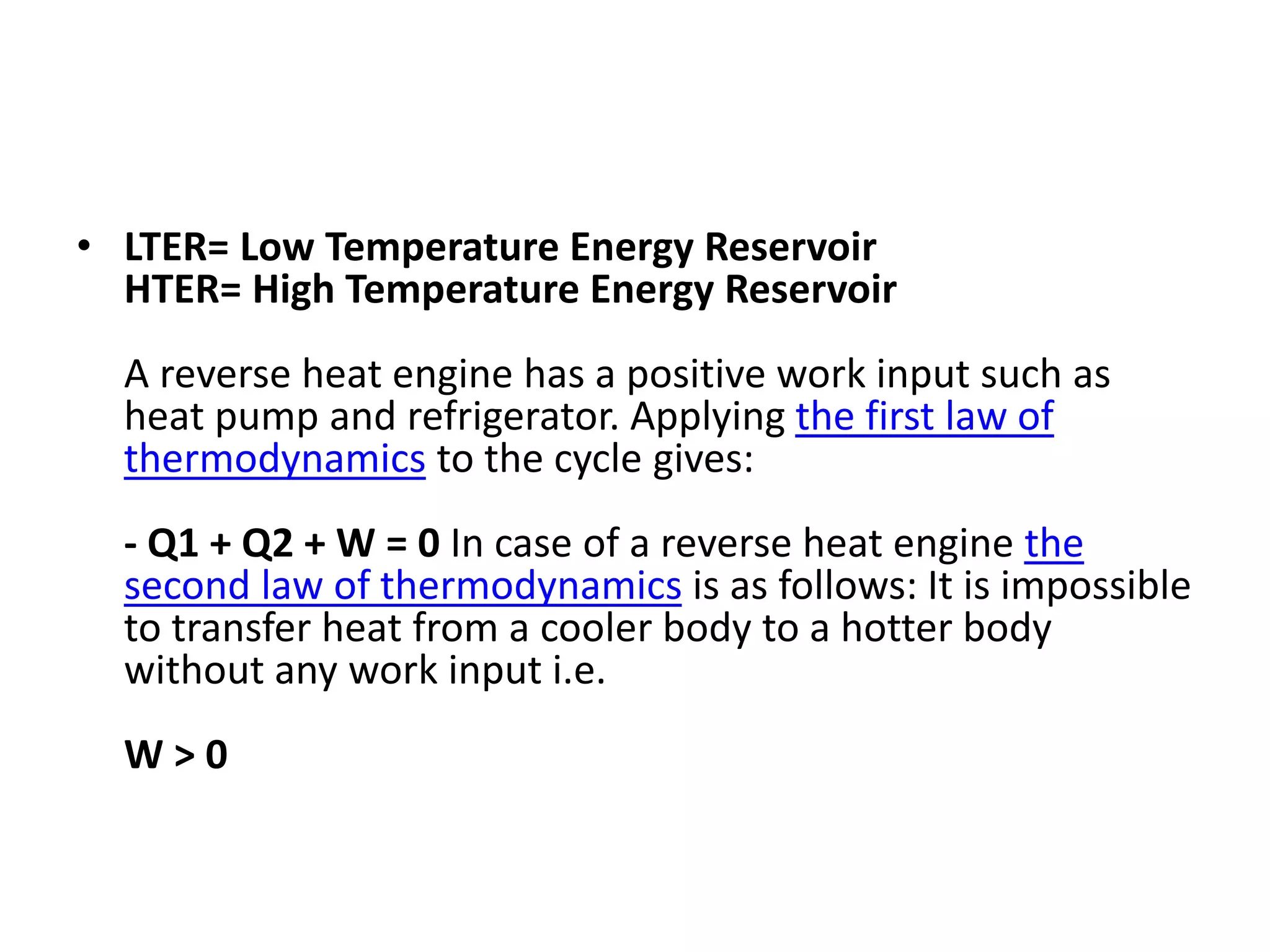
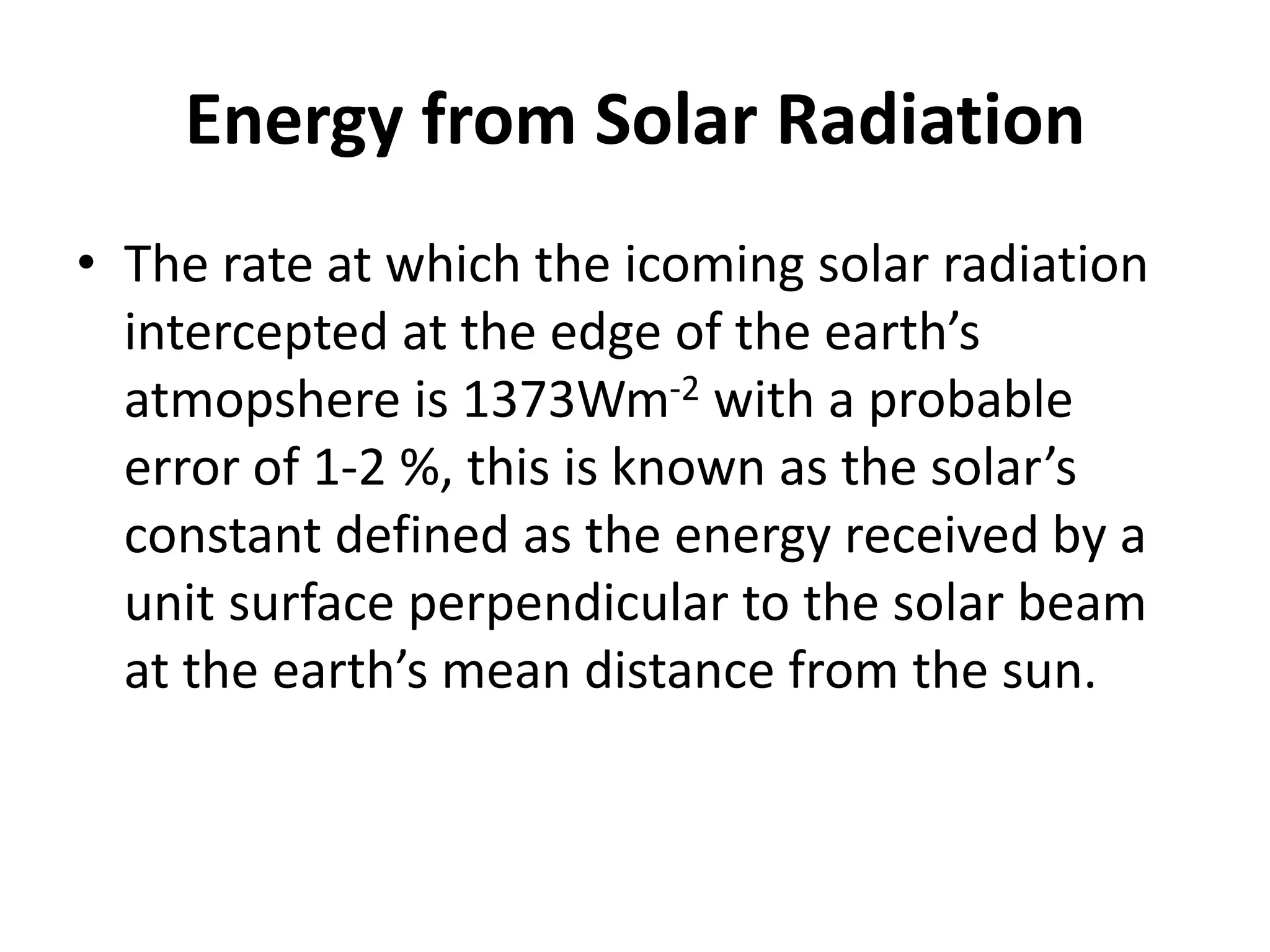
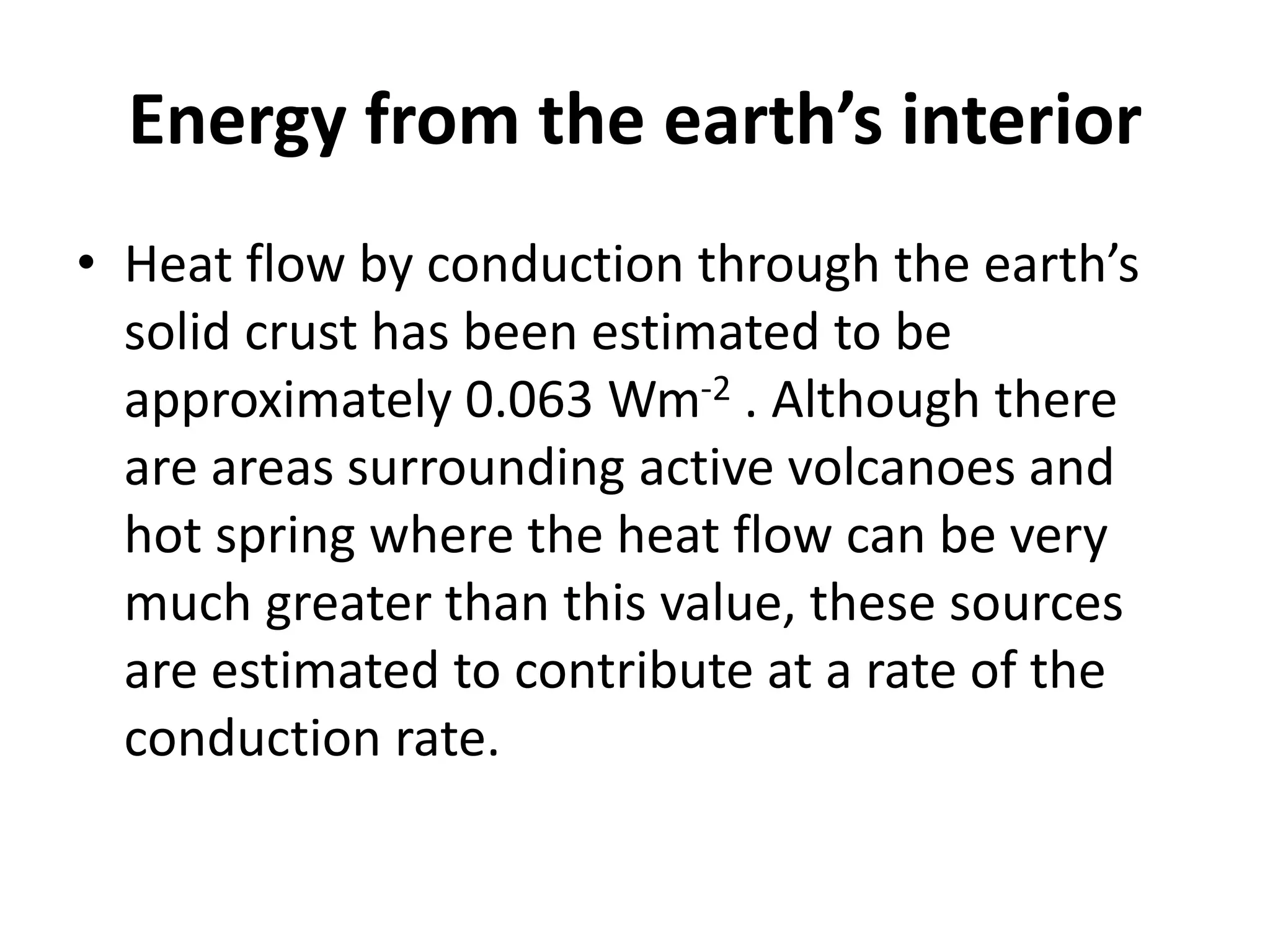
The document provides a comprehensive overview of power plants, including types such as thermal, hydroelectric, nuclear, geothermal, solar, and wind power plants. It covers essential concepts related to energy generation, thermodynamics, work, and power, detailing the principles behind various power production methods and their components. Additionally, it explores laws of thermodynamics and energy flow from various sources, emphasizing the importance of understanding these principles for effective power plant operation.