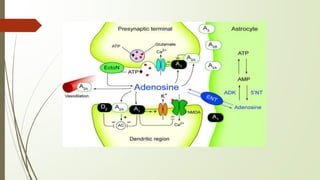
Nucleoside transporters (NTs) are membrane proteins that transport nucleosides like adenosine across cell membranes. There are two main types: concentrative nucleoside transporters (CNTs) and equilibrative nucleoside transporters (ENTs). CNTs use sodium gradients to actively transport nucleosides into cells while ENTs facilitate diffusion. Both transport naturally occurring nucleosides and synthetic nucleoside drugs. Some NTs may also act as receptors that modulate cell signaling pathways. Dysregulation of NT expression is associated with various diseases including cancer and inflammatory bowel disease.