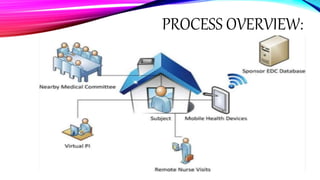
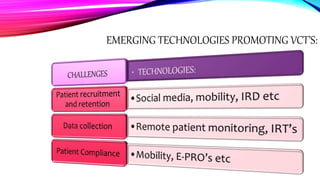
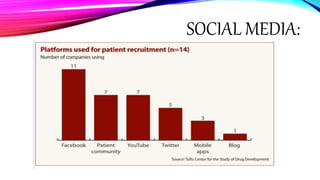
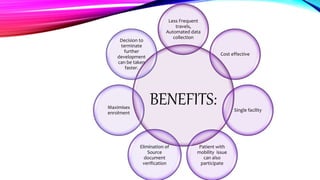
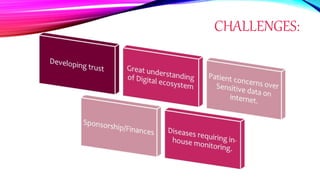
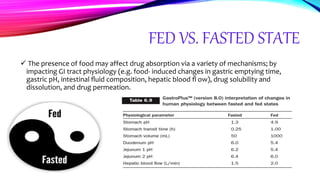
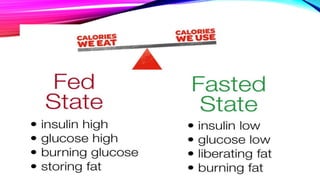
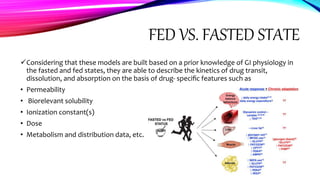
Virtual clinical trials offer advantages over traditional trials such as improved patient comfort, convenience and confidentiality. They utilize technologies like apps and online platforms to remotely collect data from trial participants from start to finish. While offering benefits, virtual trials also carry risks regarding patient privacy, operational challenges, and technical or cultural barriers. Ideal virtual trials would generate necessary data with minimal burden, foster ongoing relationships to better understand conditions, and engage providers in a complementary way. Emerging technologies like social media, mobile devices, remote monitoring, and electronic patient reporting can help promote virtual trials by automating data collection and enabling remote participation. Physiologically-based modeling using software like GastroPlus can help predict food effects on drug absorption by simulating gastrointestinal conditions