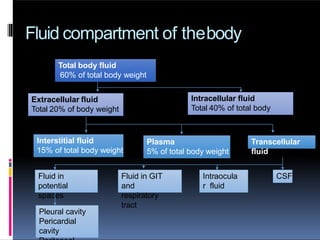
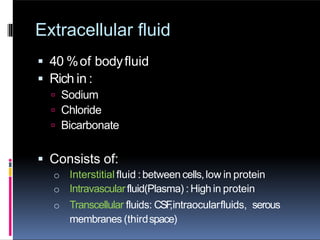
The document discusses the importance of fluid and electrolyte balance in maintaining homeostasis, detailing the composition and distribution of body fluids. It categorizes fluids into intracellular and extracellular compartments, explaining types of solutions (isotonic, hypertonic, hypotonic) and their clinical applications in surgery. Additionally, it covers fluid types such as crystalloids and colloids, including their osmolarity and electrolyte composition.