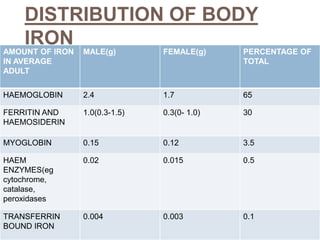
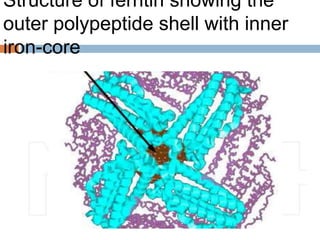
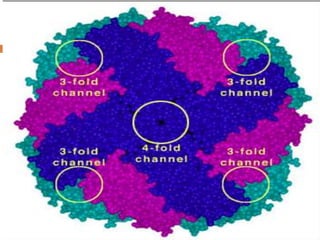
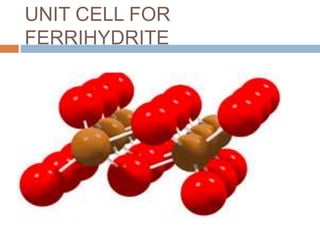
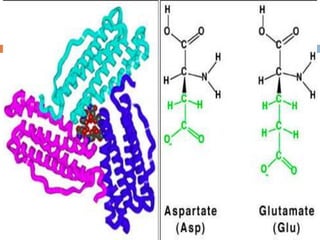
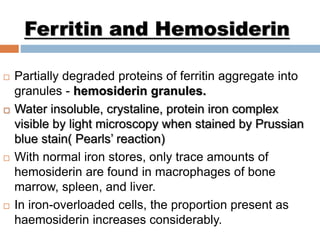
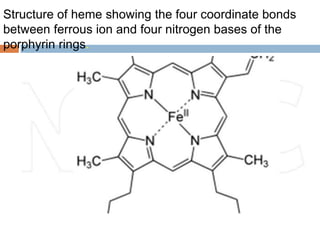
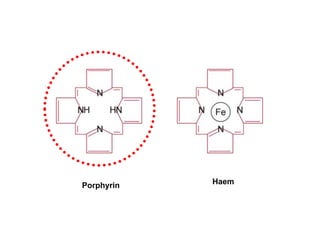
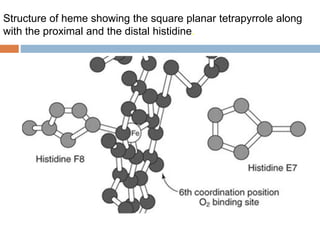
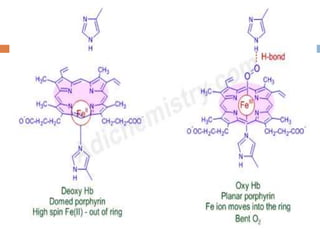
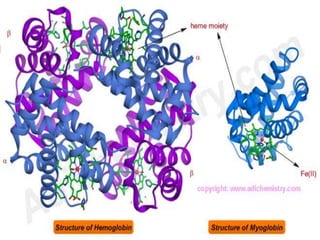
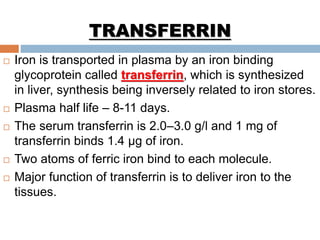
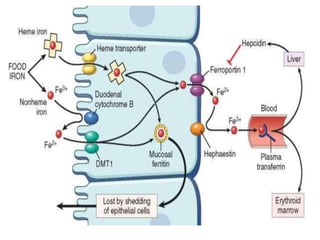
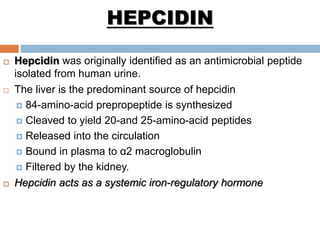
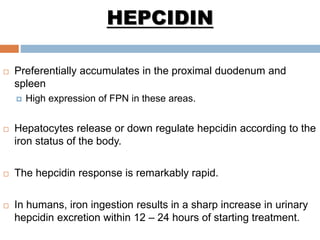
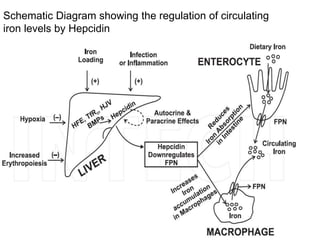
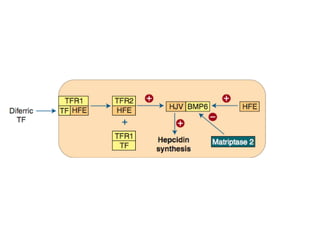
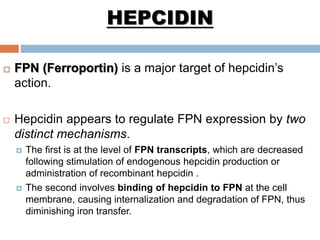
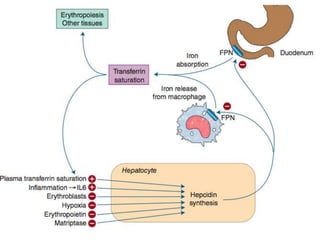
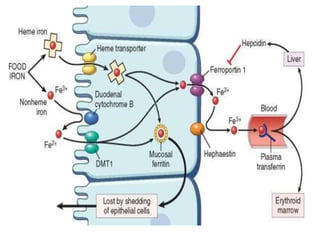
This document summarizes iron metabolism and the key proteins involved. It discusses that iron is stored in the body bound to ferritin and hemosiderin, and is transported by transferrin. Ferritin stores iron within a hollow protein shell, while hemosiderin aggregates from degraded ferritin. Transferrin transports iron in the bloodstream. Hepcidin regulates iron levels by decreasing ferroportin, the iron exporter. The document outlines the roles and structures of these iron-transport and storage proteins in maintaining iron homeostasis.