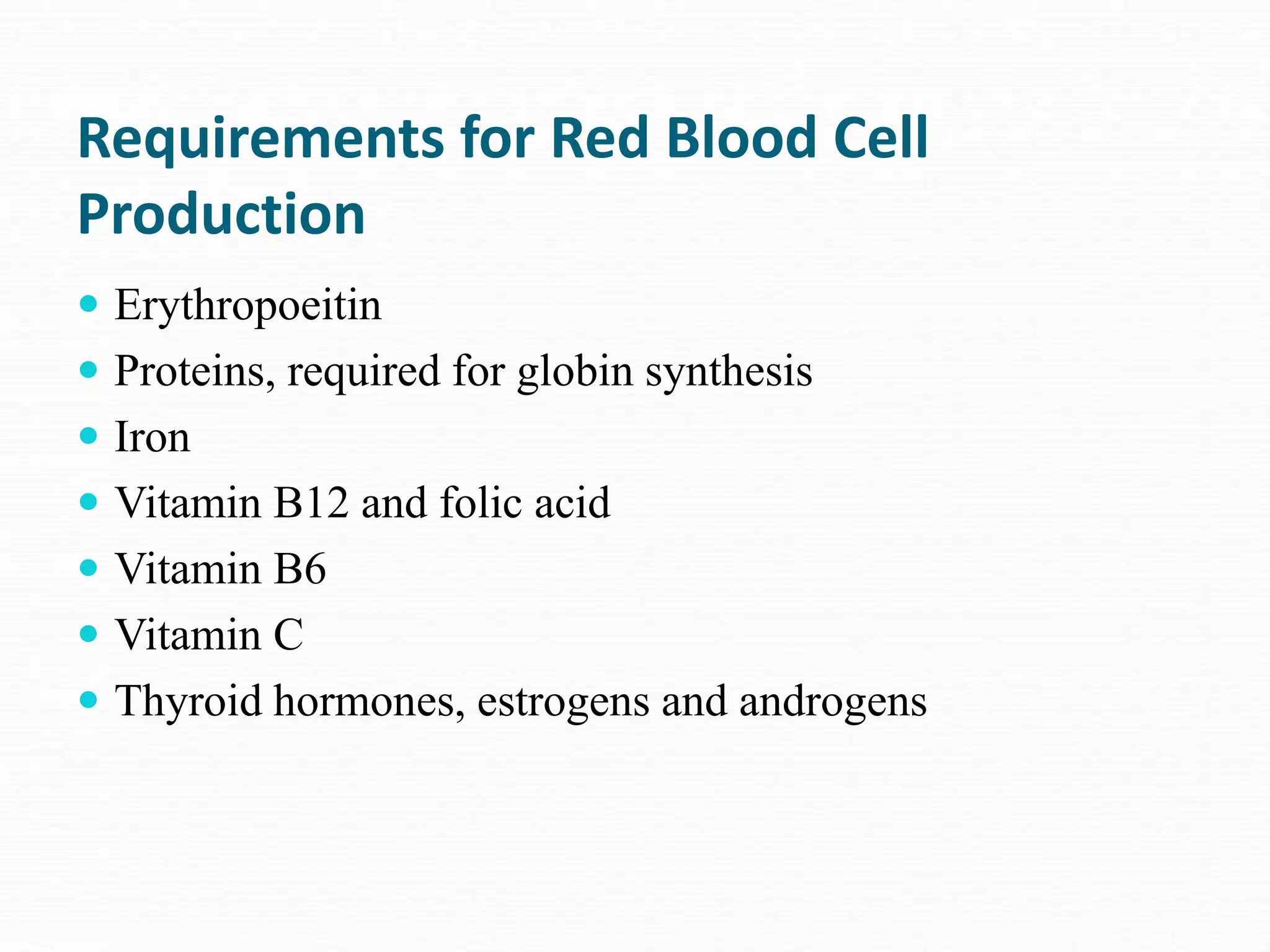
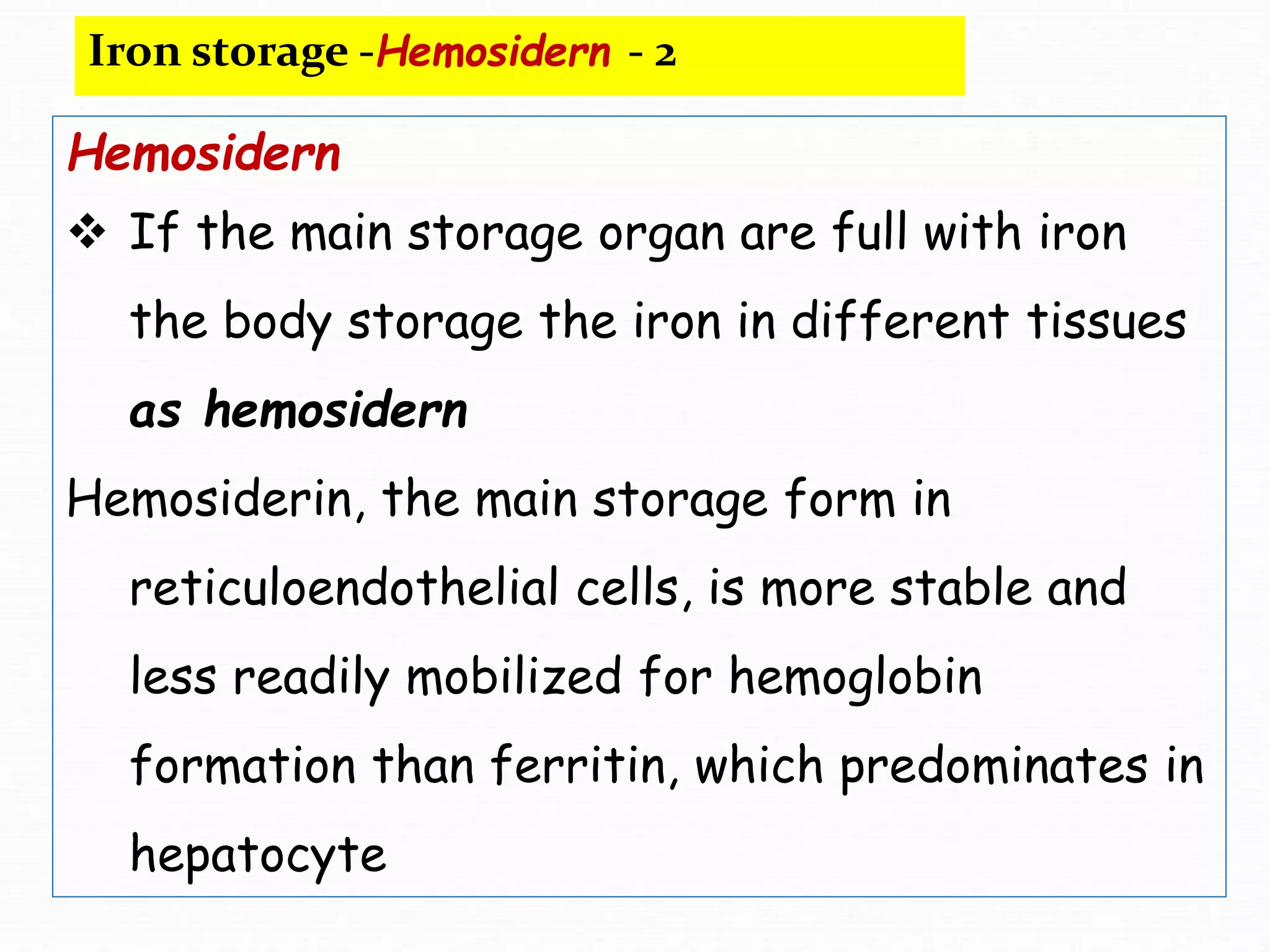
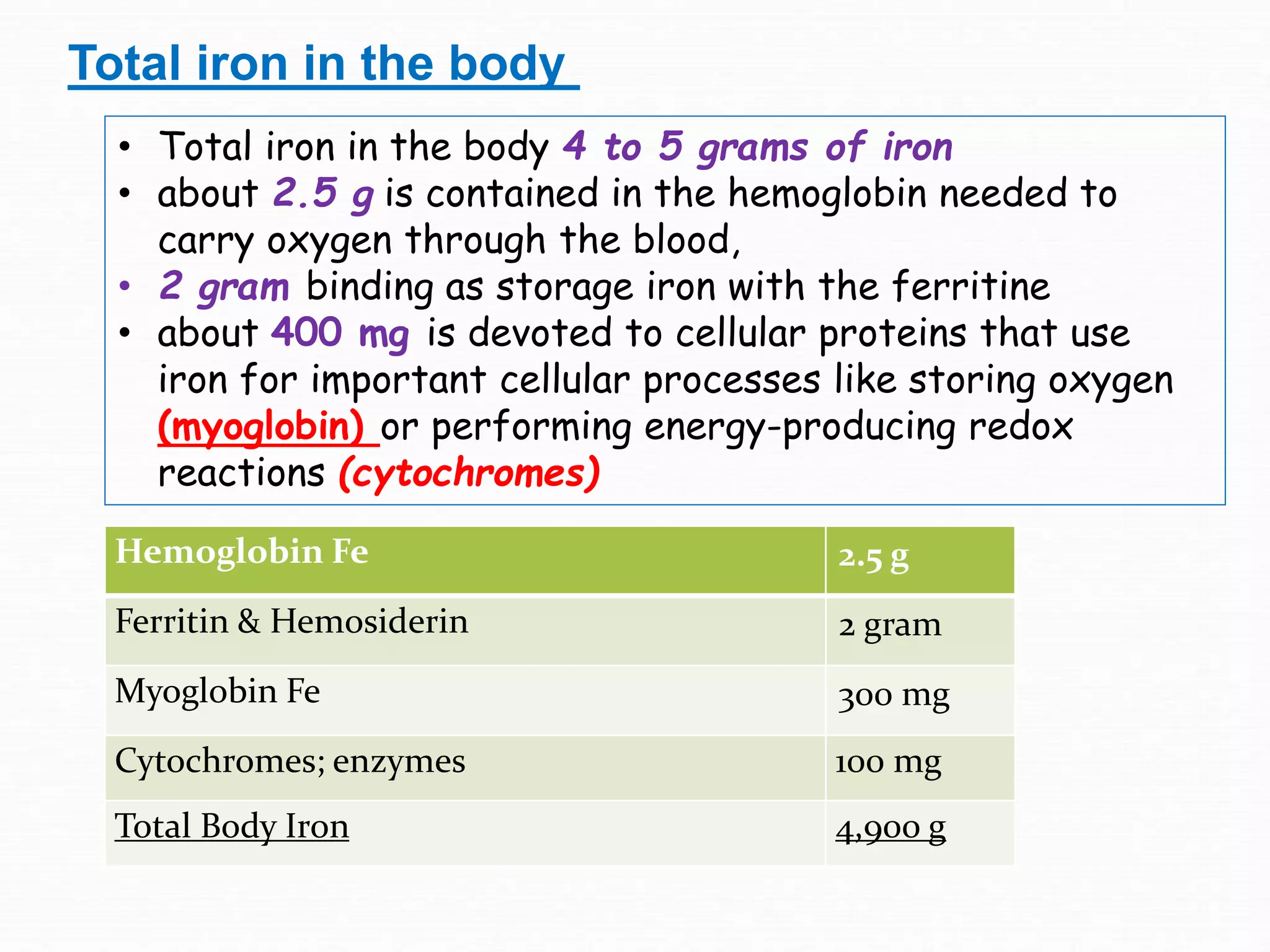
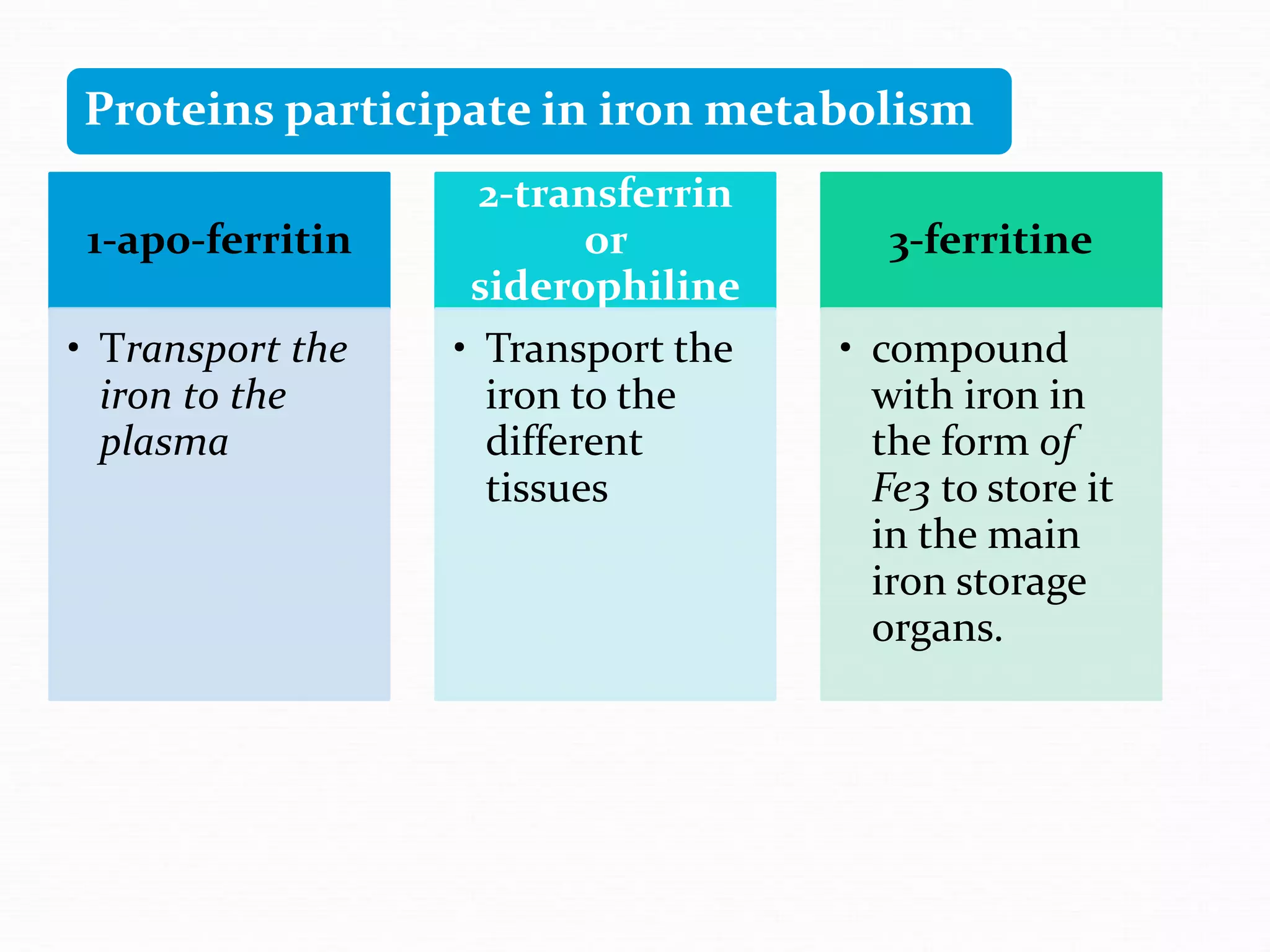
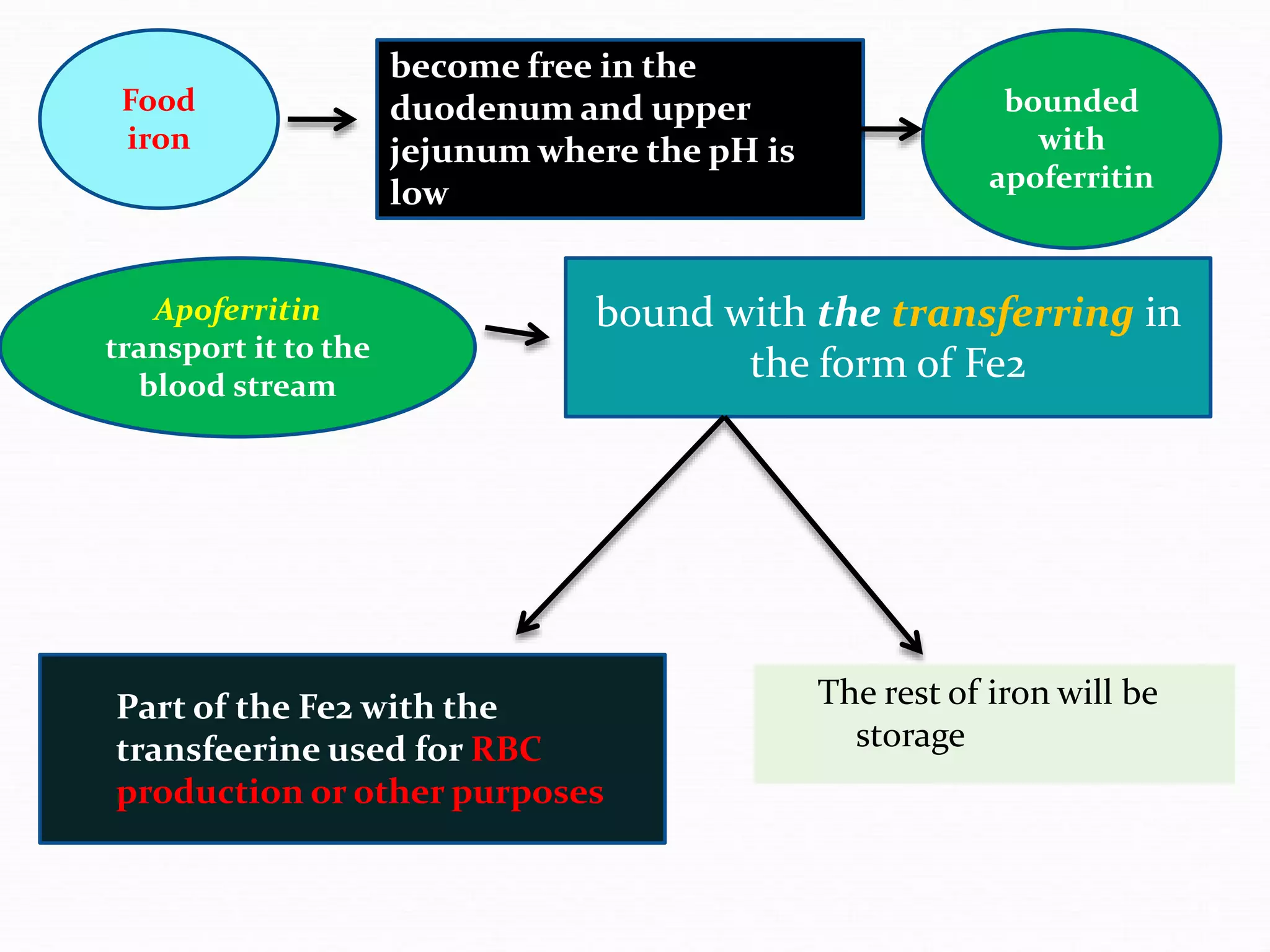
The document outlines the essential substances required for erythrocyte production, emphasizing the importance of iron and other nutrients in red blood cell formation. It details iron metabolism, including its absorption, storage, and the proteins involved in transport and regulation, as well as the daily intake and variations of iron in the body. Iron is portrayed as critical for oxygen transport and overall cellular health, with specific mechanisms in place to prevent toxicity from free iron.