1. This document summarizes iron metabolism in the human body, including sources of iron, transport and storage, and clinical aspects of iron deficiency and overload.
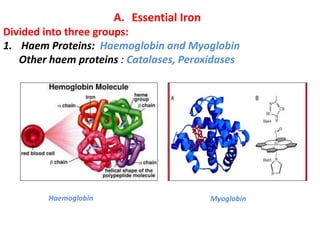
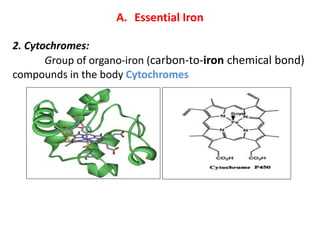
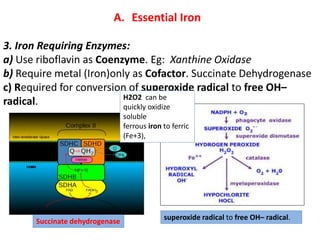
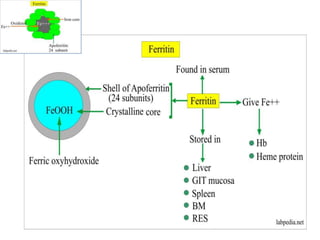
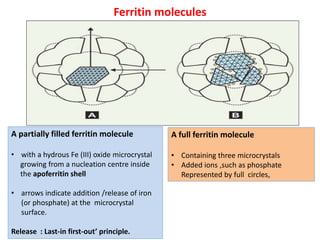
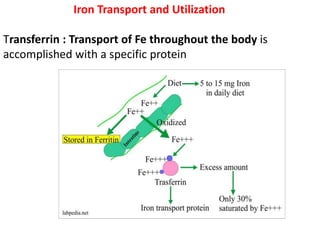
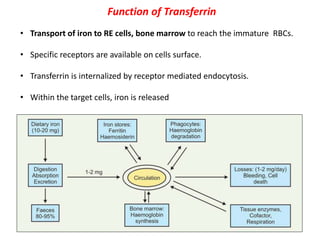
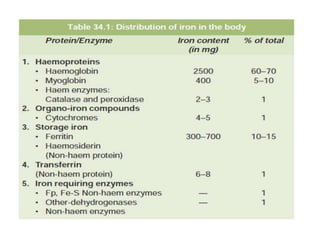
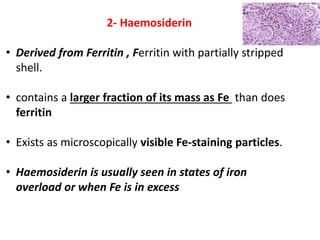
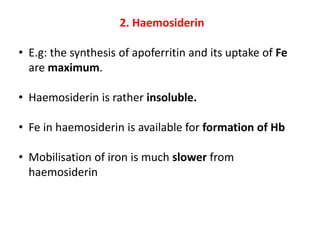
2. Iron exists in the body bound to proteins like hemoglobin, myoglobin, cytochromes and iron-requiring enzymes or stored in ferritin and hemosiderin in the liver, spleen and bone marrow. Transferrin transports iron throughout the body.
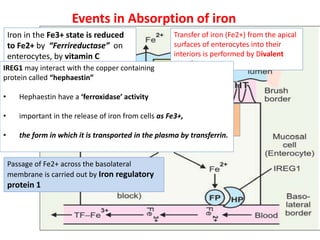
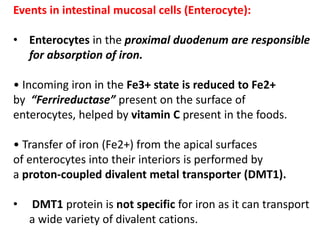
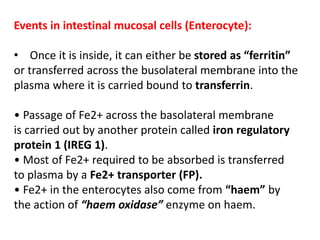
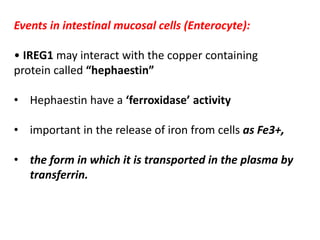
3. Iron is absorbed in the duodenum and jejunum and either stored in intestinal cells or transported into plasma bound to transferrin. Strict regulation of absorption maintains iron balance in the body.