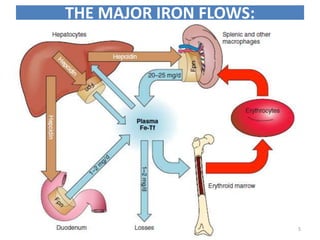
This document discusses iron homeostasis in the human body. It covers the following key points:
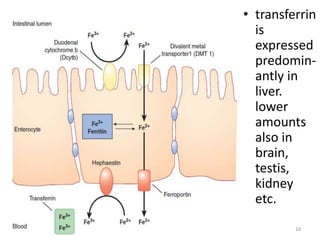
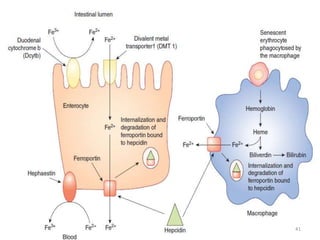
1. Dietary iron is absorbed in the duodenum and upper jejunum. It is transported across intestinal cells via DMT1 and exported into circulation by ferroportin.
2. In the circulation, iron is carried by the protein transferrin and delivered to cells via transferrin receptors that undergo endocytosis.
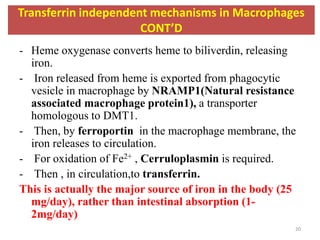
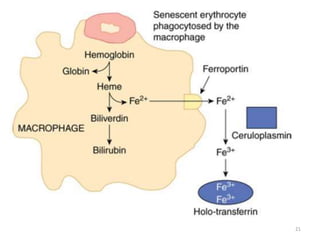
3. Iron is either used in cellular processes or stored in ferritin complexes in tissues. Iron is also recycled from senescent red blood cells by macrophages.
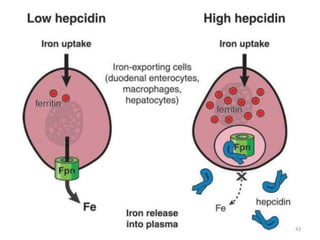
4. Tight regulation of iron absorption, transport, and storage is needed as iron is essential but also toxic in excess


![DIETARY ABSORPTION OF IRON:
• Absorbed either as a heme-iron or non-heme iron.
NON HEME IRON:
- About 90% of dietary iron
- Absorbed primarily in proximal duodenum.
- Also in upper jejunum.
- Iron absorbed is almost equal to iron lost per day
[loss through intestinal epithelial exfoliation, epidermal
sloughing, bleeding and ,in females, menstruation]
6](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-6-320.jpg)
![ABSORPTION CONT’D
- Dietary iron usually presents in ferric form.
- At epithelial surface, it is reduced to ferrous form by
Ferric reductase [ one major example being
duodenal cytochrome called dCytB]
- In several studies, disruption of dcytb, however, didn’t
show significant effect in body iron stores…So, the
existence of alternative mechanisms for iron reduction
have been suggested.
- Vitamin C is one of the important electron donor for
iron reduction, suggesting its vital role in iron
absorption.
- Gastric acids and number of other reducing agents
also play major role.
7](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-7-320.jpg)
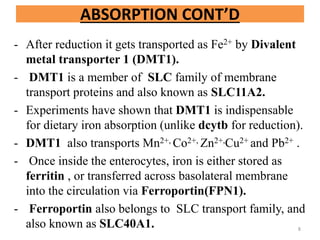
![- To transport, iron must be in ferric form.
- So, ferroportin mediated absorption occurs in conjunction
with Hephaestin,which is a membrane bound copper
containing Ferroxidase, analogous to Cerruloplasmin (which
also acts as ferroxidase, alpha 2 globulin, 160kd, synthesized
by liver).
-Now, in plasma, ferric iron is transported by transferrin.
[Apotransferrin when bound to iron is called transferrin
or holotransferrin(Tf-Fe)]
- Excess iron stored as ferritin is lost when enterocytes are
sloughed off into gut lumen.
[ Short life span of enterocytes ensure that the iron that is not
transferred to plasma is shed into fecal stream]
[Under stress condition that may exceed oxidative capacity of
Hephestin, cerruloplasmin accomplishes the oxidation of
ferrous iron to ferric] ]
ABSORPTION CONT’D
9](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-9-320.jpg)
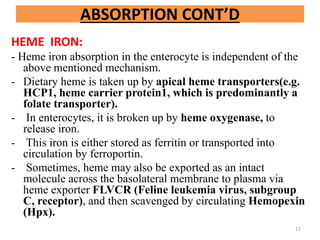
![TRANSFERRIN:
- It is a plasma protein (a glycoprotein, beta globulin..mol
wt 76 kilo dalton) that transports ferric iron in plasma.
- Free iron in plasma is extremely reactive, as illustrated
by the following Fenton reaction:
Fe2+ + H2O2 Fe3+ + OH. + OH-
[the free radical can oxidize cellular macromolecules
resulting in tissue damage]
- So, it needs a transporter transferrin (Tf).
- Tf has 2 high affinity binding sites for ferric iron.
12](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-12-320.jpg)
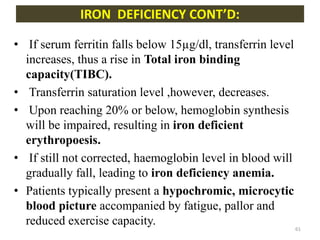
![- Plasma concentration of Tf is 300 mg/dl.
- This amount can bind approx. 300 µg/dl of iron.
- This represents Total Iron Binding Capacity(TIBC) of
plasma.
- Normally, Tf is 30% saturated.
- Saturation decreases to about 16% during severe iron
deficiency.
- Saturation is more than 45% during iron overload.
- During congenital disorders of glycosylation and
chronic alcoholism Tf fails to be glycosylated, resulting in
increased amount of CDT (Carbohydrate Deficient
Transferrin).
- [NOTE: - CDT is also an important marker for chronic
alcoholism]
TRANSFERRIN CONT’D:
13](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-13-320.jpg)
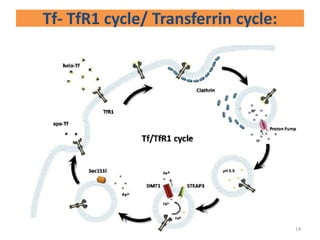
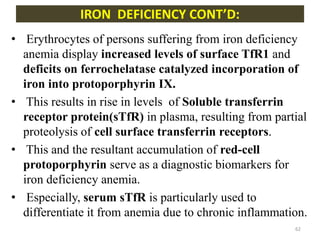
![- Transferrin Receptor 1 (TfR1) is present on the
surface of almost all cell, esp. erythroid precursors
and in the bone marrow.[high TfR1 also in
intestinal epithelial cells, placental
syncitiotrophoblast and neoplastic cells]
- TfR1 is a transmembrane glycoprotein, which forms
a disulfide bonded homodimer, which can bind 1 Tf
molecule at each of its subunit.
- When iron is added to Tf its affinity to TfR1
increases.
[ Diferric Tf has 30 times more affinity to TfR1 than
monoferric Tf…AND 500 times more than apo-Tf]
Transferrin cycle CONT’D:
15](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-15-320.jpg)
![- After holo-Tf binds to TfR1, the complex undergoes
endocytosis via Clathrin coated pits.
- Early endosome matures to form late endosome.
- The acidic pH inside late endosome (by proton pump
ATPase to pH5.5) causes iron to dissociate from Tf.
- Tf still remains bound to TfR1 inside endosome.
- DMT1 present on endosomal membrane transports the
free iron [ After it is reduced to ferrous form by ferric
reductase STEAP3(Six Transmembrane Epithelial
Antigen of prostrate3)] to cytoplasm.
- TfR1-apoTf complex is recycled back to cell
membrane.
- ApoTf is released from TfR1 .
Transferrin cycle CONT’D:
16](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-16-320.jpg)
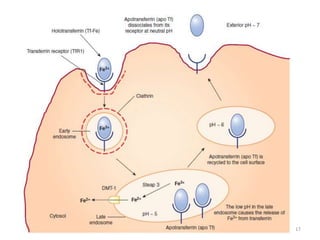
![Minor TfR1 independent mechanisms of holoTf
endocytosis have also been found
- In PCT via membrane receptor Cubilin.
- TfR2 has been expressed, mainly on hepatocytes.
However its low tissue distribution (and low affinity
to Tf compared to TfR1) doesn’t support its role in
iron uptake.
[MORE OF TfR2 later]
- GADPH (Glyceraldehyde 3 Phosphate
dehydrogenase) and proteoglycans have also been
reported to mediate endocytosis of holoTf in
macrophages and hepatocytes
[NOTE: -TfR2 not expressed in intestinal crypt cells]
18](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-18-320.jpg)
![- Ferritin only takes ferrous iron, which is oxidized to
ferric form by a catalytic site on the H chain.
- H chains also contain small intra sub unit channels that
facilitate entry of iron into storage cavity of the
molecule.
- The exact composition of FeOOH core crystal varies
according to species, and may also contain some amount
of phosphates.[3000-4500 ferric atoms in 1 ferritin]
- In humans it is ferrihydrite (Fe2O3.9H2O).
- Function of L chain isn’t properly known (proposed to
play a role in ferritin nucleation and stability).
ABOUT FERRITIN CONT’D:
23](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-23-320.jpg)

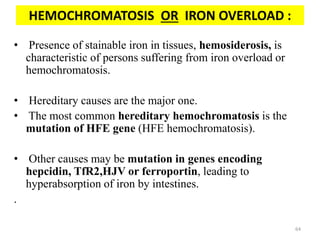
![- It is an aggregated, partially deproteinized ferritin that is
formed when ferritin is partially degraded in secondary
lysosomes.
- While ferritin is soluble, it is insoluble in aqueous
solutions [which forms traditional basis of
distinguishing these 2 proteins]
- From hemosiderin, iron is only slowly released.
- Detected in tissues under condtions of iron overload
(hemosiderosis), by histological stains (e.g. prussian
blue).
HEMOSIDERIN:
25](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-25-320.jpg)

![- Now, the iron enters mitochondria.[Where it will be
utilized in various enzymatic processes of cellular
metabolism]
- Mitochondrial entry is by yet another transporter,
Mitoferrin[Mfrn, also a member of SLC transporter
…AKA SLC25A37]
- Several experiments in hemoglobin synthesizing
erythroid cells have also provided evidence that iron can
be directly transported from endosome to the
mitochondria via ‘kiss and run’ mechanism, where 2
organelles exchange iron when in direct contact.
CELLULAR IRON METABOLISM CONT’D:
28](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-28-320.jpg)
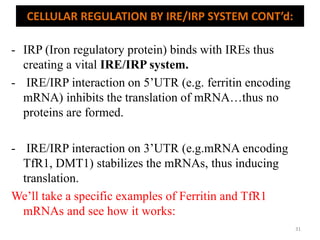

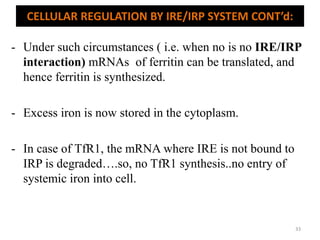
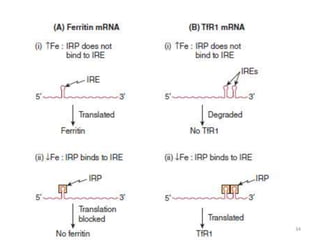
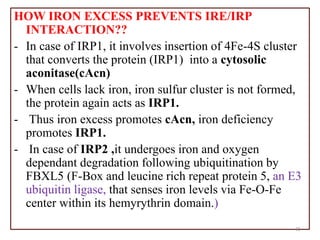



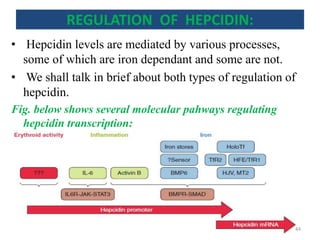

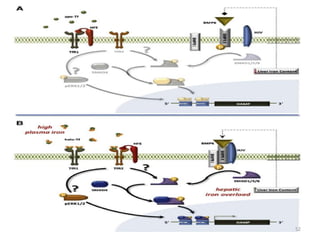
![An important example of HFE regulation of hepcidin:
- Abbreviation for High Fe [AKA HUMAN
HEMOCHROMATOSIS PROTEIN].
- It is a Major Histocompatibility class 1 (MHC class1) like
molecule that is expressed on the cell surface, bound to β2
microglobulin and TfR1.
- Mice with severe disruption of either HFE or TfR2 have
been found to develop iron overload due to hepcidin
suppression.
- HFE hemochromatosis is actually the most prevalent form
of hereditary hemochromatosis.
So, there should be some interplay between HFE, TfR1,
TfR2 and hepcidin.
46](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-45-320.jpg)
![- HFE competes with holotransferrin(Tf-Fe) for TfR1.
- When there is excessive iron overload, HFE is freed from
TfR1.
- The displaced HFE now binds to TfR2.
[NOTE: TfR2 may bind with both HFE and holo Tf at once.
Holo-Tf (and not apo-Tf) actually stabilizes TfR2.]
- HFE- TfR2 interaction complex activates signaling to
hepcidin expression .
[NOTE:-However, some independent effects of HFE and TfR2
on hepcidin regulation (besides these) have also been
suggested because Mice with deficient HFE and TfR2
developed more severe iron overload than mice with either
HFE or TfR2 deficiency.]
An important example of HFE regulation of hepcidin
CONT’D:
47](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-46-320.jpg)
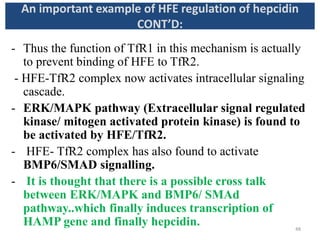
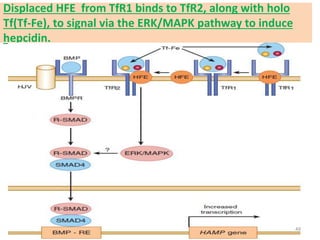
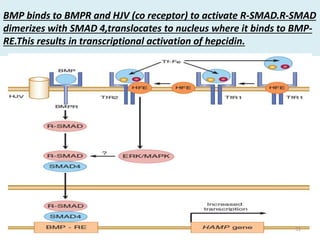
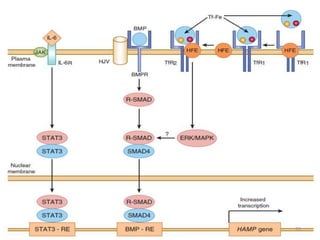
![BONE MORPHOGENIC PROTEIN (BMP, esp. BMP6) AND
HEPCIDIN REGULATION
• BMP6/SMAD pathway of hepcidin regulation is a
major pathway of hepcidin regulation.
• Increased hepatic iron is thought to induce hepcidin
expression via this pathway.
• HFE-TfR2-holoTf is also thought to enter the
intermediate of this pathway for hepcidin regulation.
The core thing is the binding of BMP to BMPR leading
to phosphorylation of SMAD(intracellular signalling
protein)
[Hemojuvelin (HJV) acts as the co-receptor of BMPR and
genetic mutation to form this protein forms the basis for
iron overload condition called HJV Hemochromatosis]
50](https://image.slidesharecdn.com/ironhomeostasis-190208124432/85/Iron-Homeostasis-49-320.jpg)