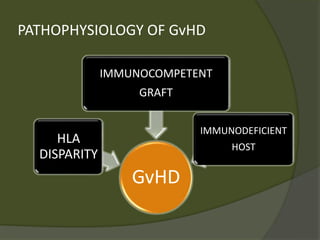
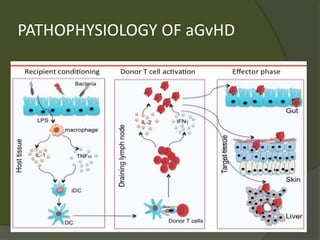
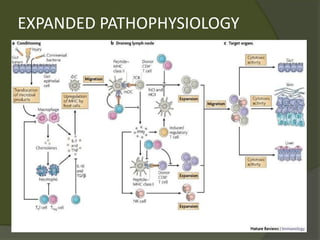
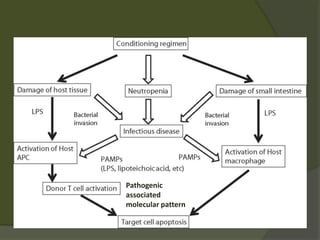
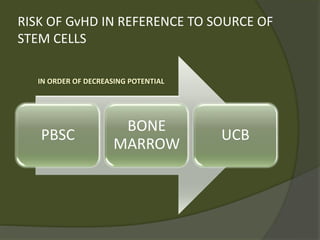
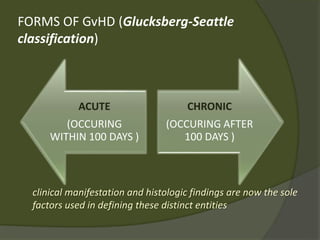
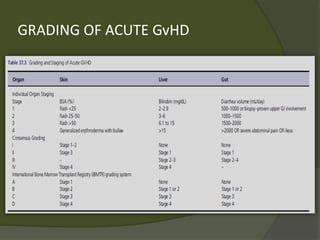
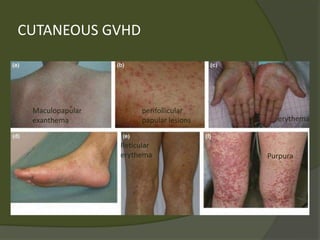
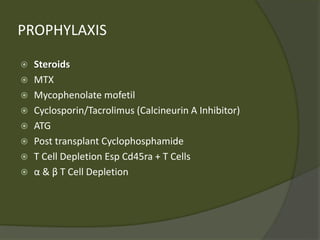
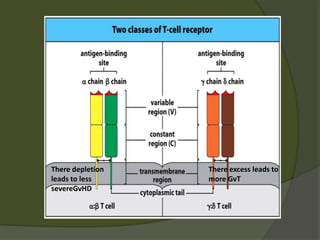
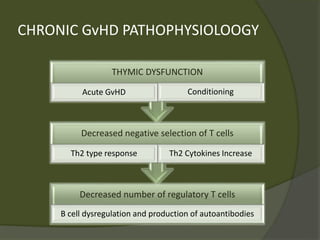
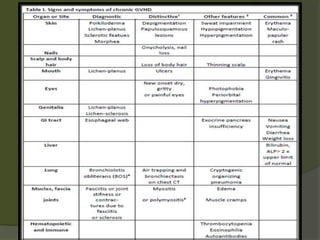
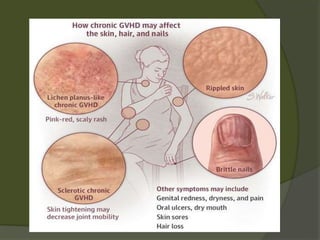
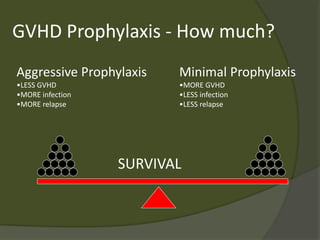
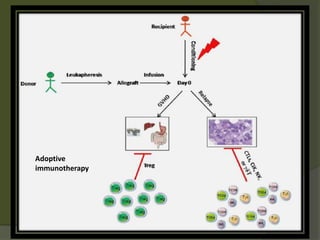
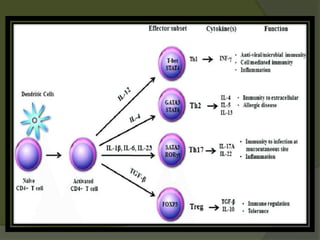
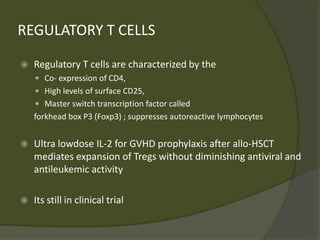
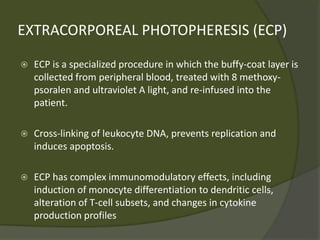
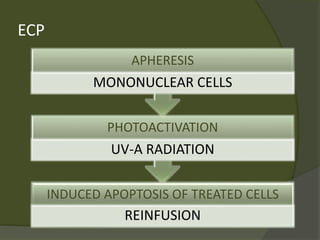
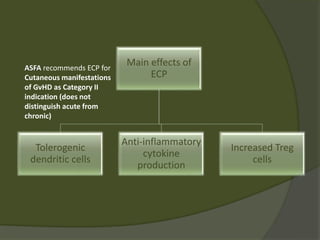
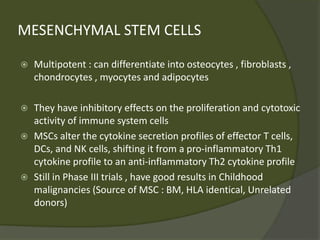
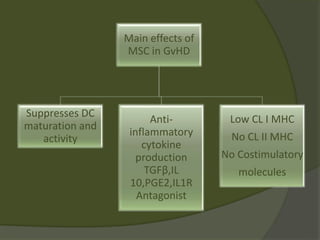
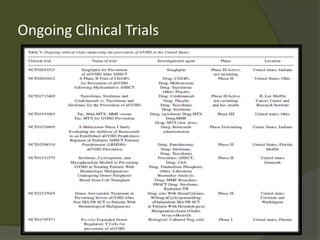
The document discusses graft-versus-host disease (GVHD) as a significant complication following hematopoietic stem cell transplantation (HSCT), detailing its causes, risk factors, and effects on patient outcomes. It highlights the role of HLA compatibility in reducing GVHD incidence and underscores the importance of monitoring and managing complications to improve survival rates. Advances in therapies such as adoptive immunotherapy and extracorporeal photopheresis are also reviewed, emphasizing the ongoing challenges in understanding and mitigating GVHD.