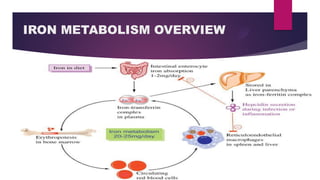
1) Iron is an essential trace element that is stored and transported throughout the body by heme-containing and non-heme proteins.
2) Ferritin and hemosiderin are the primary proteins involved in iron storage in the liver, bone marrow, and spleen. Ferritin stores iron in a soluble form while hemosiderin stores excess iron in an insoluble aggregate.
3) Transferrin is the main protein responsible for transporting iron through the blood plasma. It binds iron released from ferritin and transports it to tissues where iron is utilized or stored.