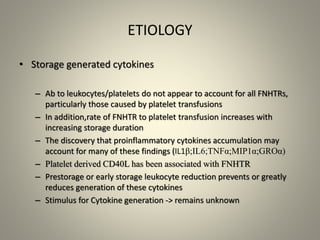
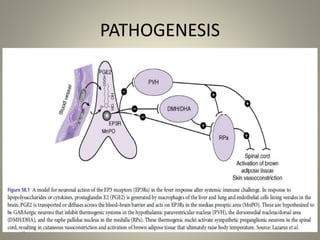
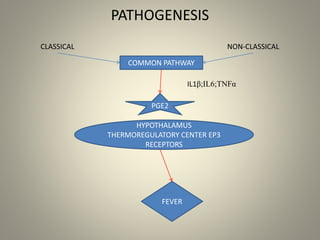
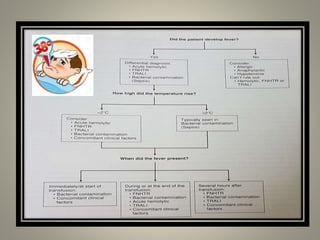
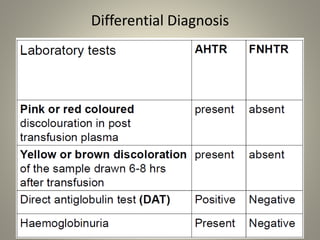
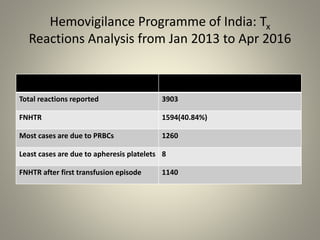
- Febrile non-hemolytic transfusion reactions (FNHTRs) are the most frequently reported transfusion reactions, occurring in 0.1-1% of transfusions. They are caused by cytokines and inflammatory mediators released from leukocytes in the transfused blood components.
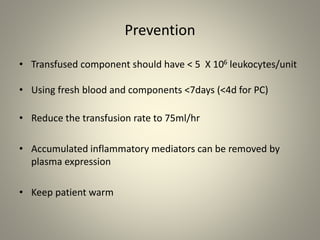
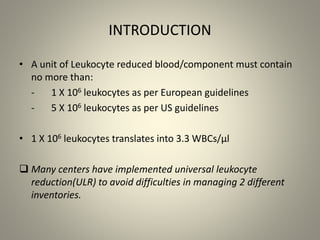
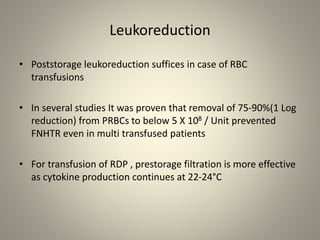
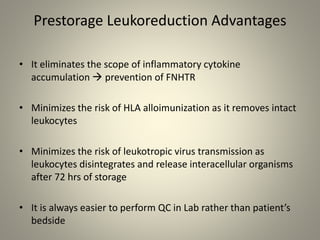
- Leukoreduction, which reduces the white blood cell count to less than 1x10^6 WBCs/unit, is an effective prevention strategy as it reduces the sources of inflammatory cytokines. Prestorage leukoreduction is preferred over bedside filtration as it prevents cytokine accumulation during storage.
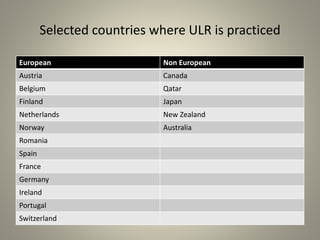
- Universal leukoreduction has been adopted by many countries and healthcare facilities due to its proven clinical benefits in reducing transfusion reactions and