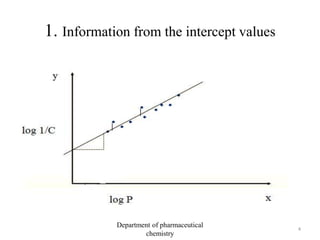
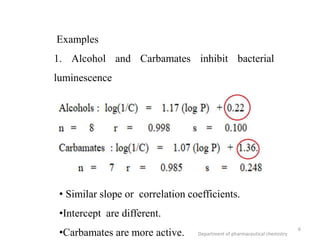
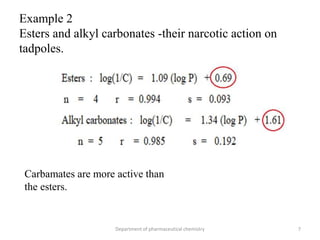
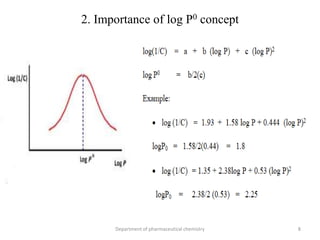
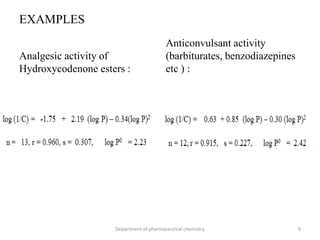
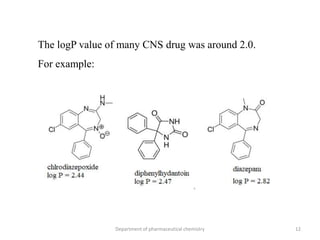
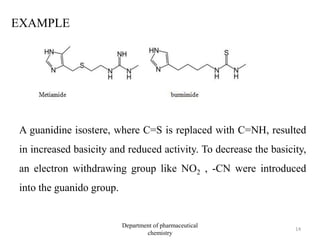
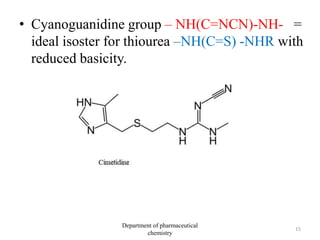
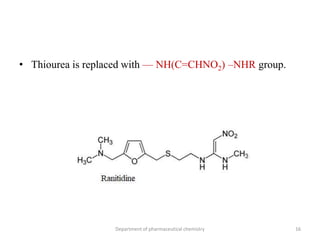
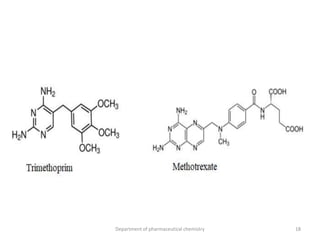
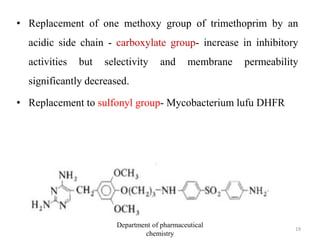
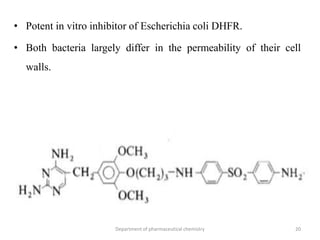
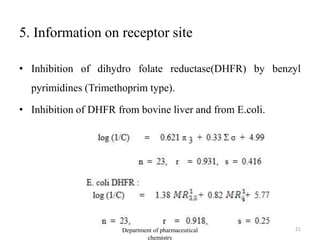
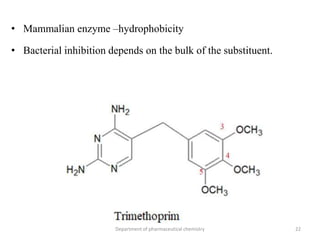
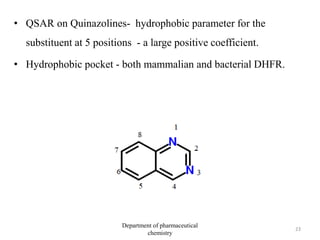
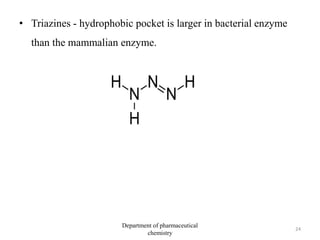
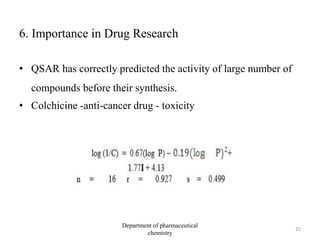
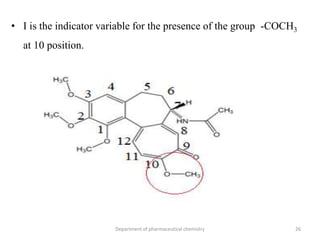
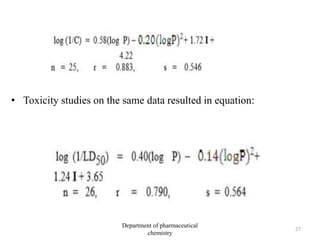
This document discusses various applications of quantitative structure-activity relationships (QSAR) in drug research and development. It describes how QSAR can provide information on receptor sites from enzyme inhibition studies, help understand the importance of physicochemical properties like log P values, and enable the use of bioisosterism to modify compounds. QSAR has correctly predicted drug activity and toxicity and is useful in drug design before compounds are synthesized.