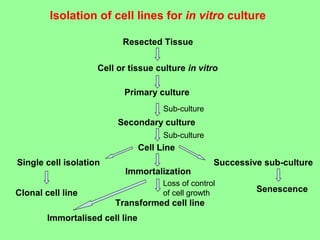
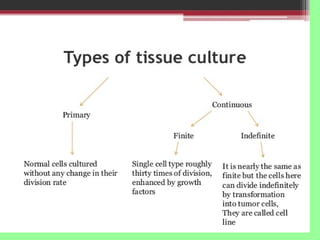
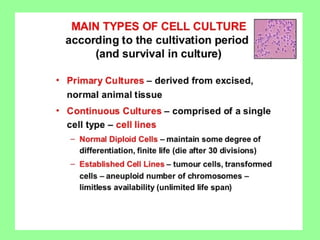
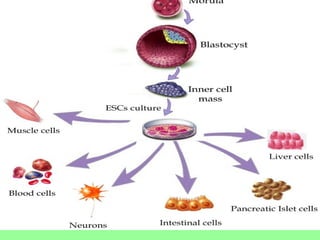
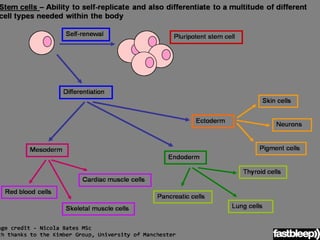
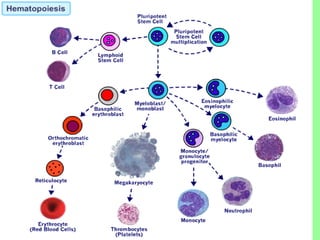
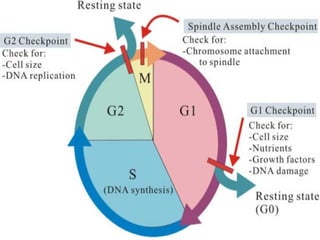
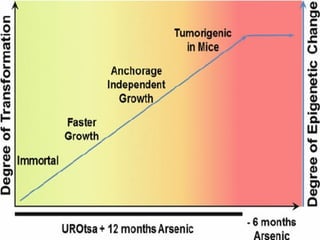
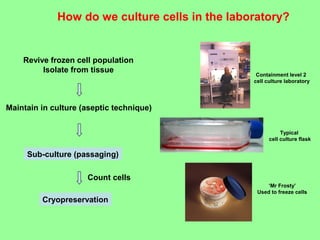
- Cell culture techniques have developed significantly since 1885 when embryonic chick cells were first maintained alive in saline solution for short periods. Key developments include producing continuous cell lines in 1943 and defining specific growth factor requirements in the 1970s.
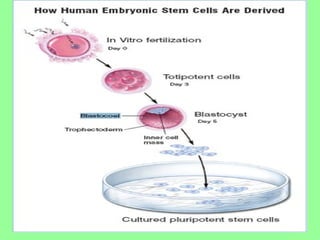
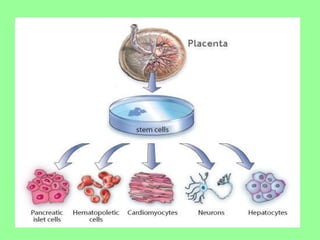
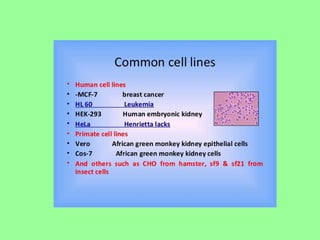
- Cell culture is now a widely accepted research tool with applications like studying cell behavior without animal variations, artificial organ development, vaccine development, and drug testing while avoiding ethical issues of animal experimentation.
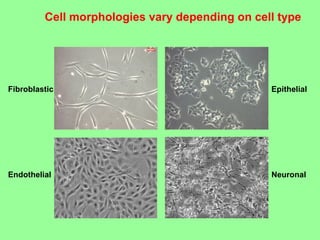
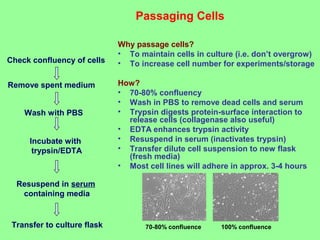
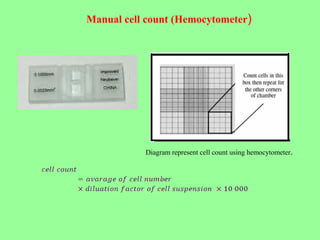
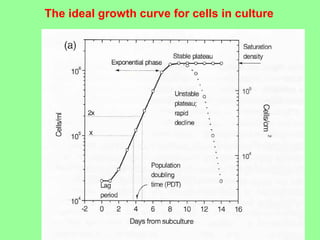
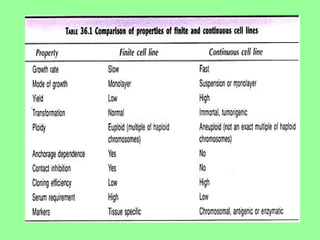
- Maintaining healthy, reproducible cells requires standardized techniques and controlling contamination remains important.