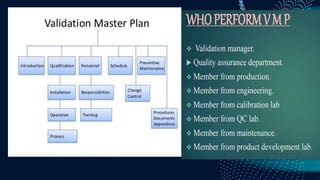
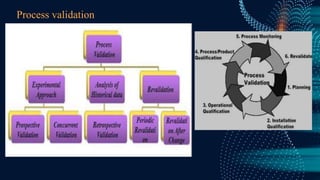
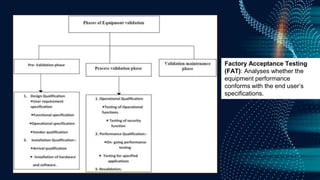
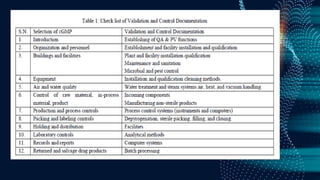
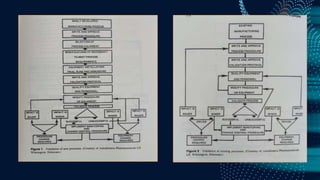
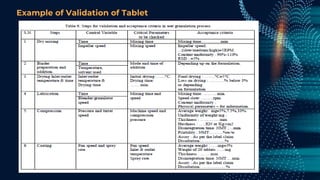
The document discusses pharmaceutical validation, including definitions, types, and elements of validation. It provides definitions of validation from WHO, FDA, and ICH. The main types of validation discussed are process validation (prospective, concurrent, retrospective), analytical method validation, equipment validation (design qualification, installation qualification, operational qualification, performance qualification), and revalidation. The key elements of validation discussed are specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. Validation is presented as an important part of ensuring consistent and quality pharmaceutical production.