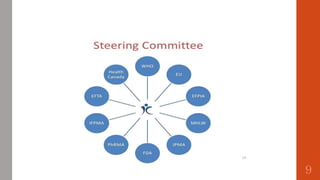
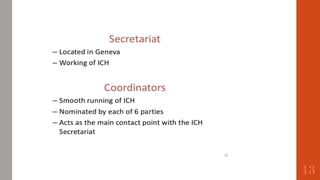
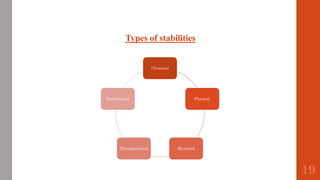
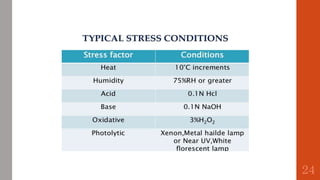
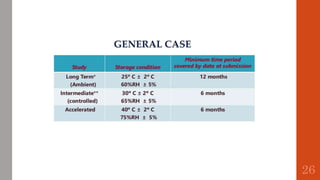
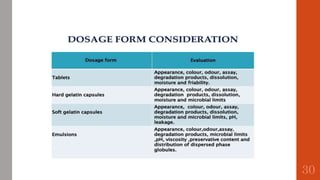
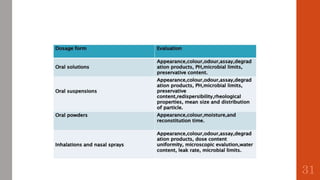
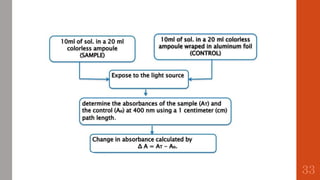
The document outlines the International Conference on Harmonisation (ICH), established in 1990 for the purpose of harmonizing global pharmaceutical regulations among the EU, Japan, and the USA. It emphasizes objectives such as ensuring the safety and efficacy of medicines, minimizing unnecessary clinical trials, and providing guidelines for quality, safety, and efficacy. Additionally, it details various stability testing guidelines and analytical validation processes critical for drug registration and quality assurance.