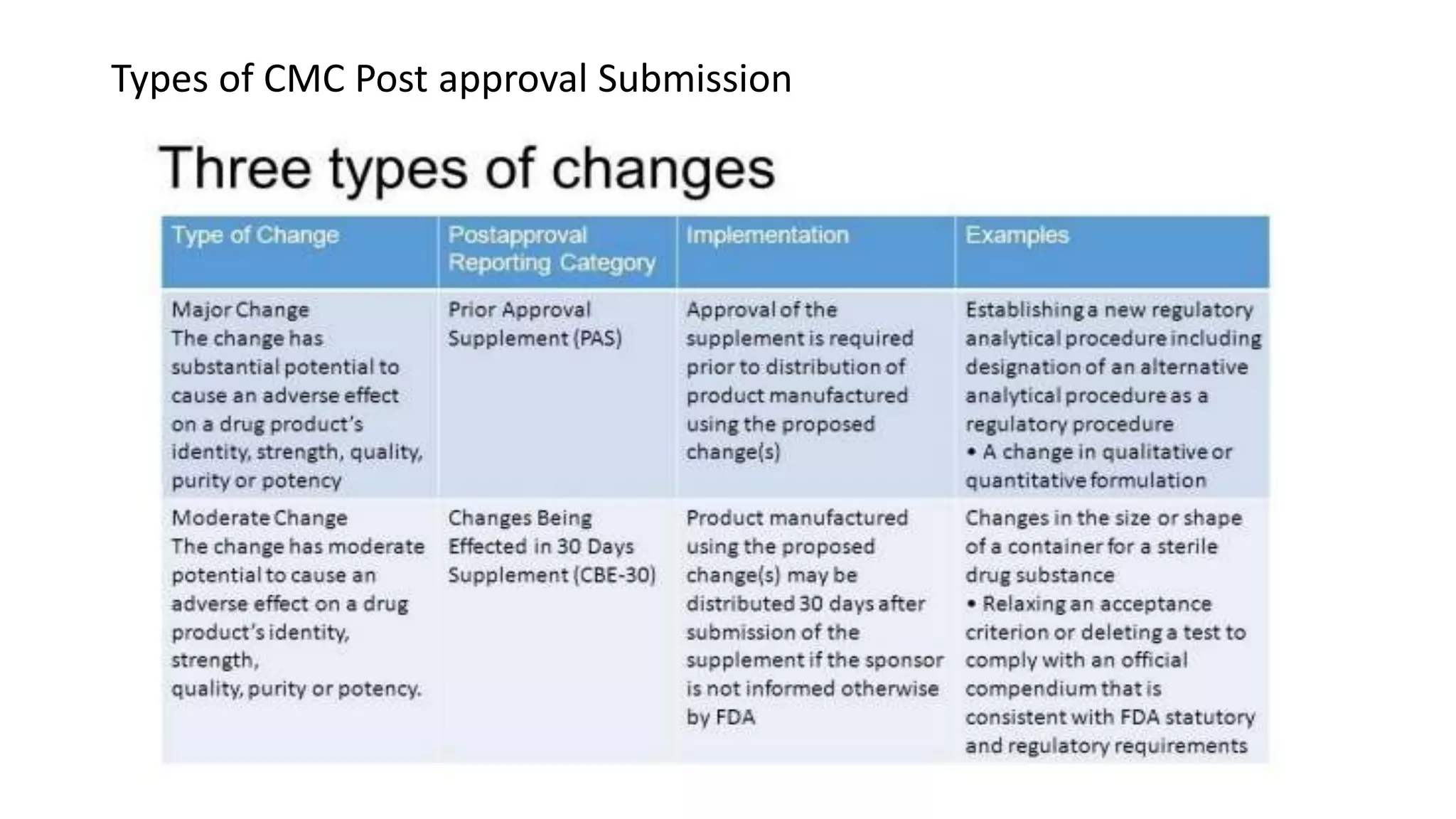
The document discusses post-approval regulatory affairs. It notes that the FDA may require post-approval studies to ensure continued safety and effectiveness of approved drugs and devices. Failure to comply with post-approval requirements can result in withdrawal of approval. It also discusses requirements for making and reporting manufacturing and distribution changes to approved applications, including prior approval supplements, changes being effected supplements, and annual reports. Finally, it provides examples of major, moderate, and minor labeling changes that may require different levels of regulatory submission.