The document provides details on Master Formula Records (MFRs), including that MFRs:
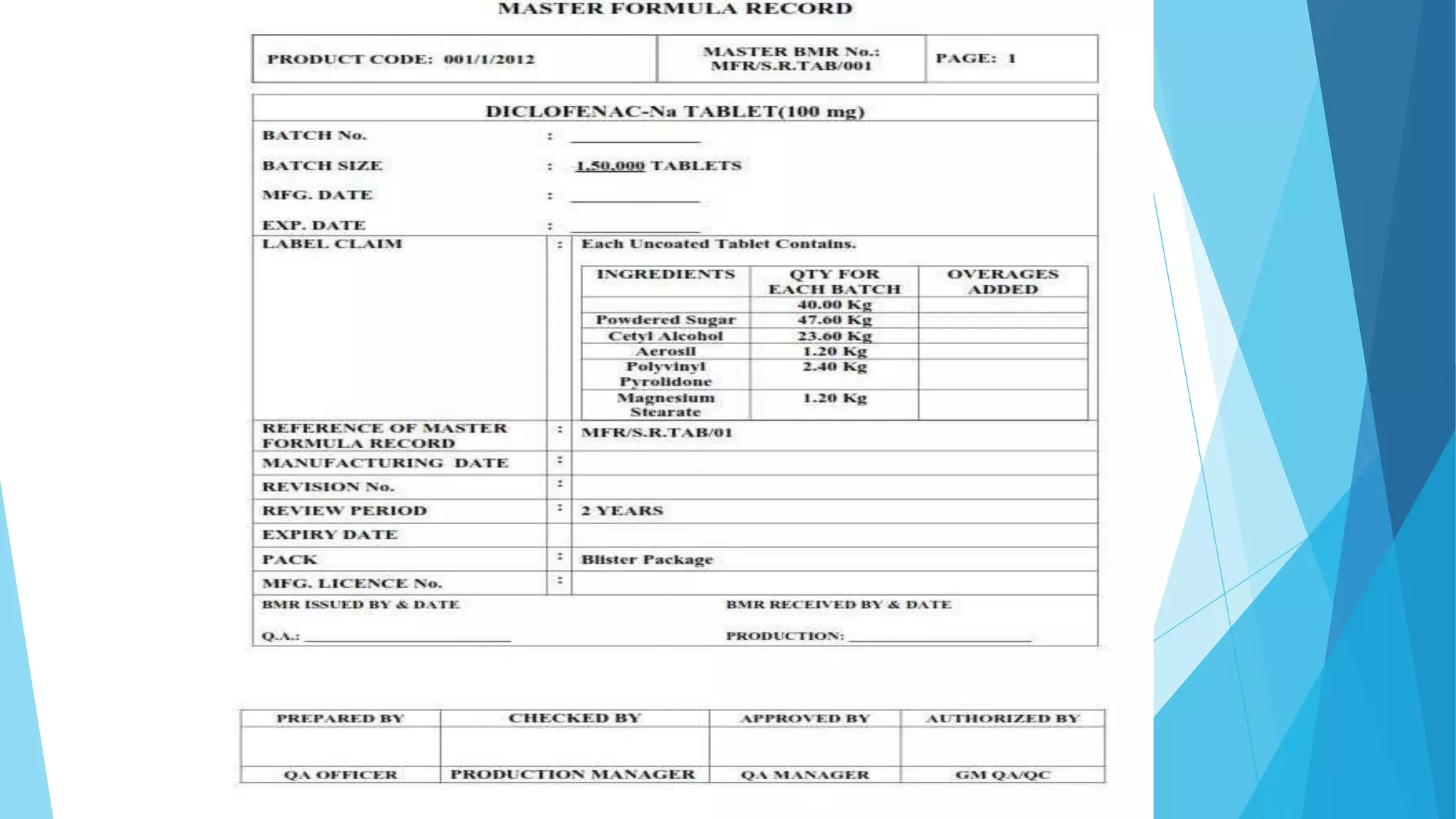
- Are master documents that contain all information about manufacturing a pharmaceutical product, including ingredients, quantities, processes, and quality checks.
- Are prepared by the research and development team and used as a reference for batch manufacturing.
- Include product details, manufacturing processes, packaging processes, calculations, and quality approval by production and quality assurance heads.
MFRs ensure consistency in production and provide standards for Batch Manufacturing Records. Comprehensive information in MFRs allows for accurate reproduction of manufacturing batches.

![MASTER FORMULA RECORD [MFR]
Master Formula Record (MFR) is a master document for any
pharmaceutical product.
MFR contains all information about the manufacturing process
for the product.
MFR is prepared by the research and development team of the
company.
MFR is used as reference standard for preparing batch
manufacturing record (BMR) by manufacturing units.
MFR is also called Master Manufacturing Record,
Master Production Record.](https://image.slidesharecdn.com/mfrnotes-210301114753/75/MFR-2-2048.jpg)