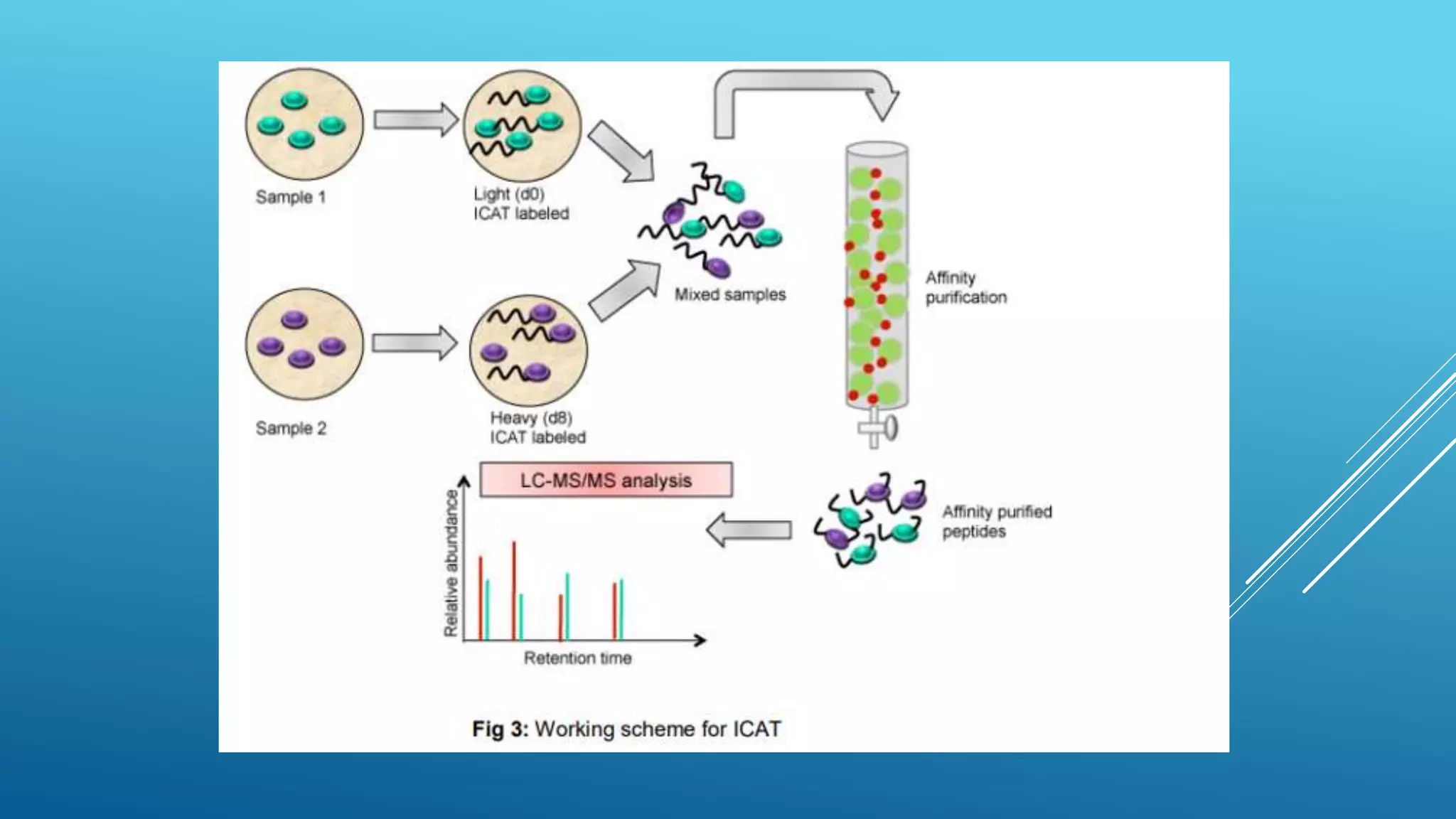
This document provides an outline of a lecture on the ICAT technique for quantitative proteomics. It begins with definitions of ICAT labeling and terminology used. ICAT labels peptides or proteins at cysteine residues with either light or heavy isotopic tags. The light tag contains hydrogen while the heavy tag contains deuterium. Tagged peptides are then purified using streptavidin affinity chromatography. The document discusses the need for gel-free proteomics due to limitations of gel-based methods. It provides an overview of the ICAT working protocol, which involves labeling proteins or peptides with light and heavy tags, trypsin digestion, affinity purification, and MS analysis to determine expression ratios.