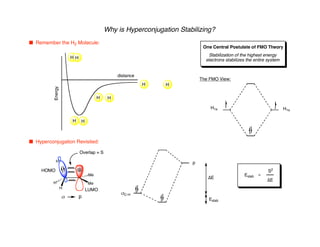
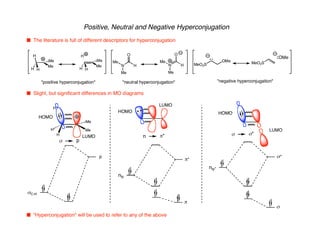
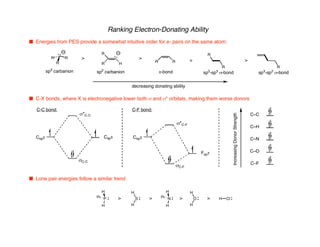
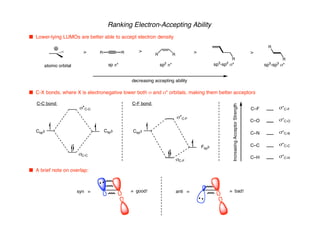
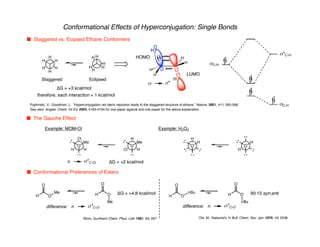
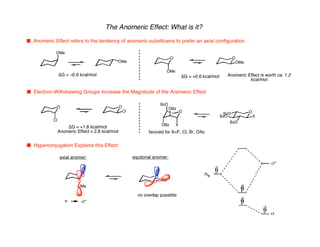
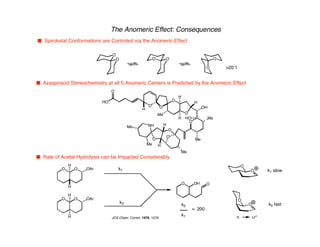
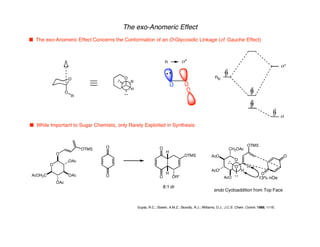
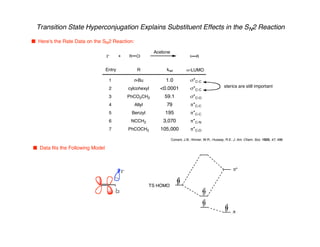
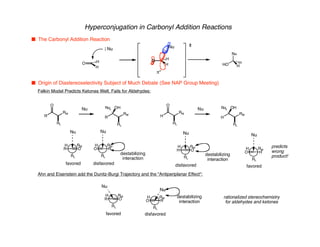
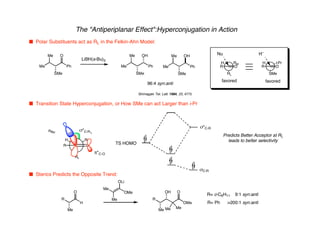
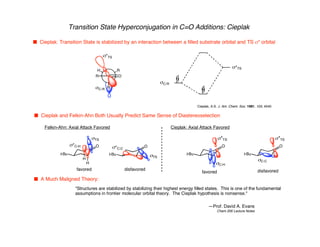
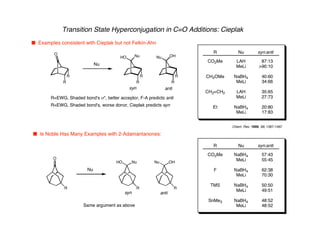
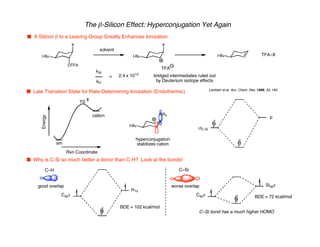
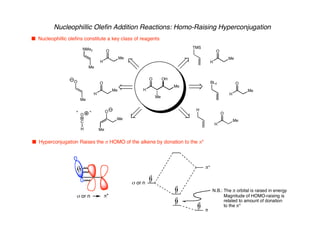
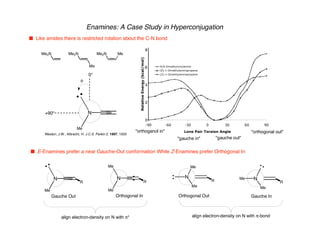
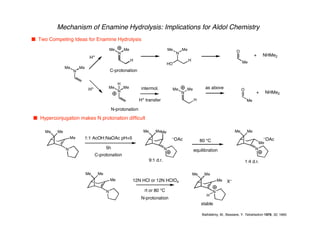
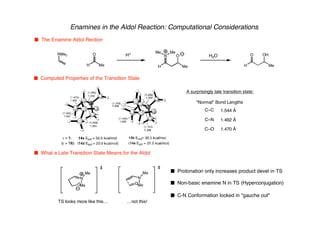
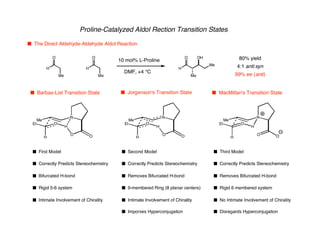
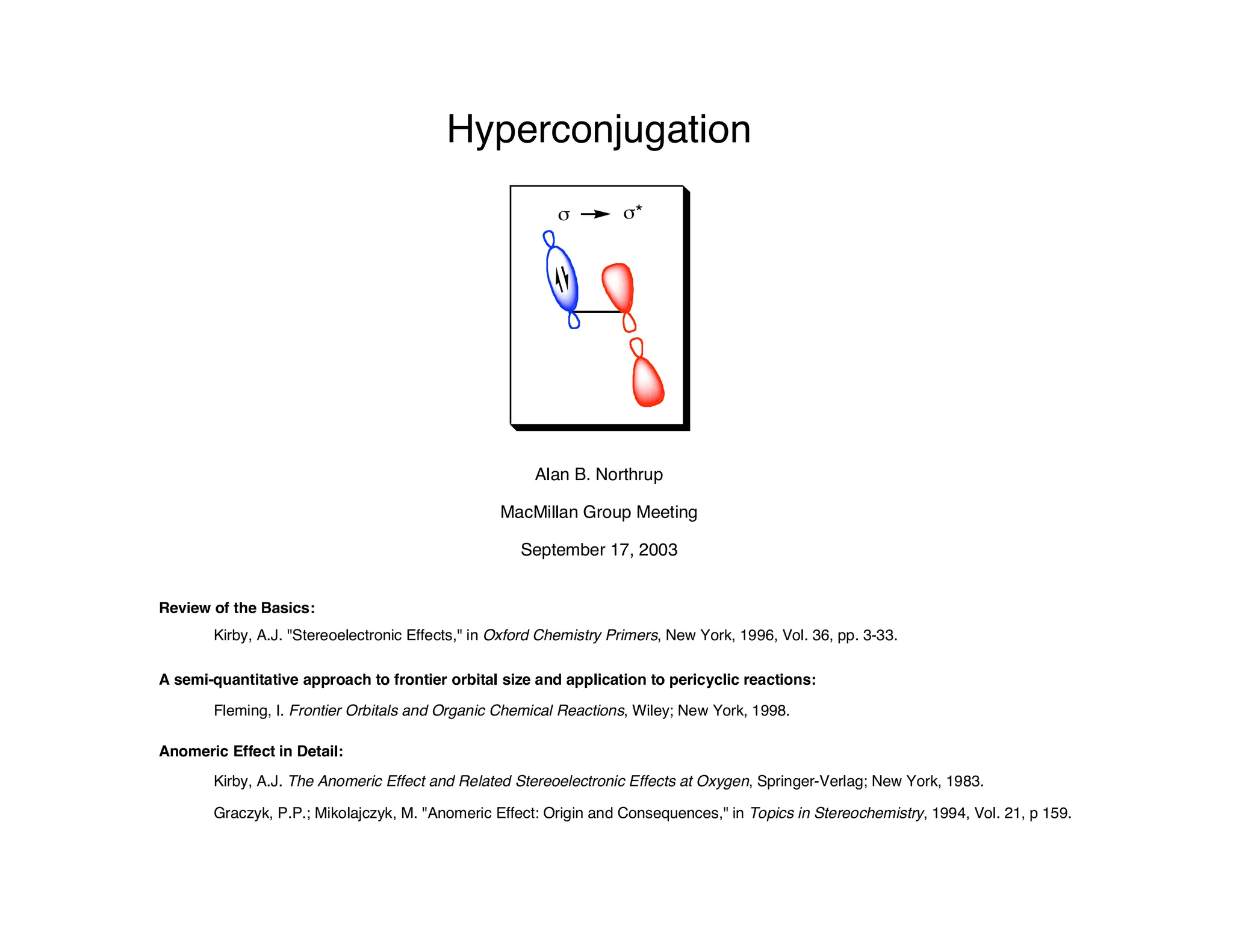
Hyperconjugation refers to stabilization that occurs through overlap between occupied bonding orbitals and unoccupied antibonding orbitals. It is observed in single bonds, double bonds, and transition states. Hyperconjugation explains conformational preferences, reaction rates, and stereochemical outcomes. The anomeric effect, where an axial orientation is favored at anomeric centers, arises from hyperconjugative interactions between the lone pair and antibonding orbital. Substituent effects in SN2 reactions and carbonyl addition reactions can be understood through stabilization of developing partial charges in the transition state by hyperconjugation.
![s p
What is Hyperconjugation?
n A Resonance View:
Me
Me
H
H H
H
Me
Me
H H
n Frontier Molecular Orbital Depiction:
H
H
Me
Me
H
HOMO
LUMO
sC-H
p
∆E
Estab
Overlap = S
Estab a
S2
∆E
n Physical Evidence for Hyperconjugation's Existance:
Adamantane
110° 1.530 Å
[SbF5–F–SbF5]–
Adamantyl Cation X-Ray Struture
100.6°
1.431 Å
1.608 Å
Laube, T., et al. Angew. Chem. Int. Ed. 1986, 25, 349](https://image.slidesharecdn.com/hyperconjugation-240331093831-ad6afef4/85/hyperconjugation-notes-of-bsc-chemistry-2-320.jpg)