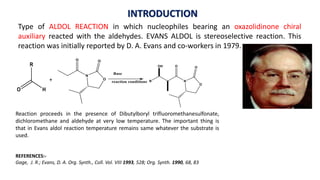
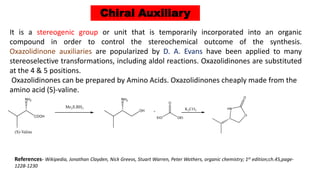
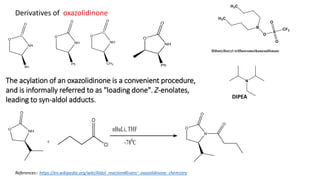
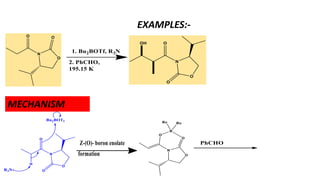
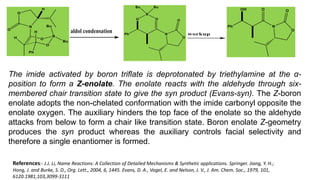
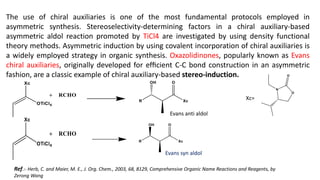
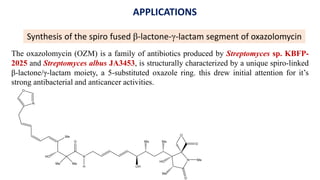
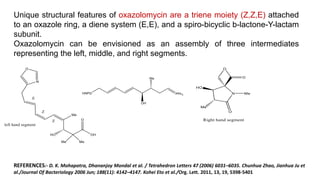
The document discusses Evans' aldol reaction, which uses chiral oxazolidinone auxiliaries to control stereoselectivity. The reaction was developed by D.A. Evans in 1979 and involves a nucleophilic oxazolidinone reacting with an aldehyde. The oxazolidinone auxiliary is temporarily incorporated to induce chirality and controls the facial selectivity of the reaction. The mechanism proceeds through a Z-enolate intermediate to give the syn product stereoselectively. Evans' aldol has been widely used in asymmetric synthesis, particularly of natural products.