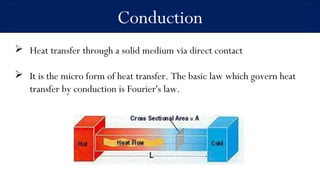
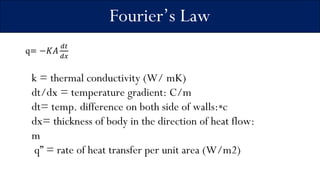
1) The document discusses three main modes of heat transfer: conduction, convection, and radiation. Conduction involves heat transfer through direct contact within a solid medium. Convection refers to heat transfer by fluid motion in either forced or natural situations. Radiation transfers heat through space or matter without conduction or convection.
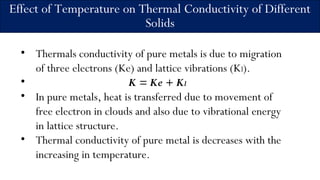
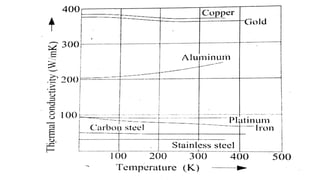
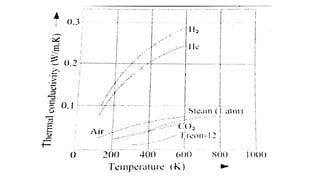
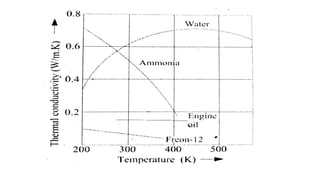
2) It describes how the thermal conductivity of solids, liquids, and gases is affected by temperature changes. The thermal conductivity of most solids and liquids decreases with increasing temperature, while the conductivity of gases generally increases with temperature.
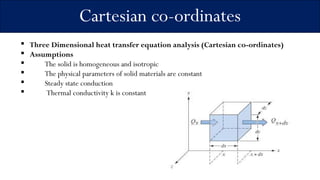
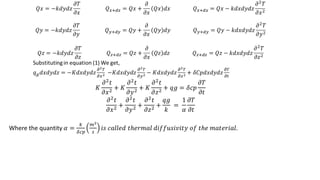
3) The document derives a generalized heat transfer equation for three-dimensional Cartesian coordinates, assuming a homogeneous, isotropic solid with constant physical parameters under steady-state conduction