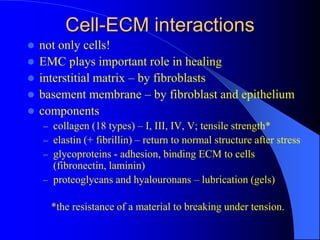
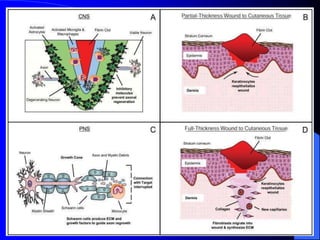
The document discusses various aspects of tissue healing and repair, including regeneration and repair processes, types of tissues involved, and cell-ECM interactions critical for tissue regeneration. It highlights processes like angiogenesis, granulation tissue formation, and the differences in healing mechanisms between neural and non-neural tissues, along with pathological aspects of healing such as keloids. The document also covers bone repair mechanisms in both primary and secondary remodeling, and the formation of glial scars in the CNS.