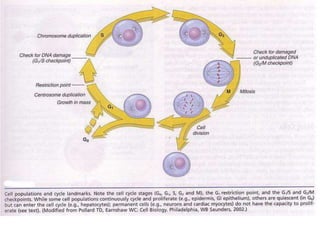
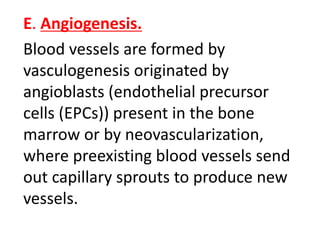
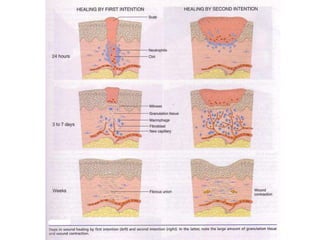
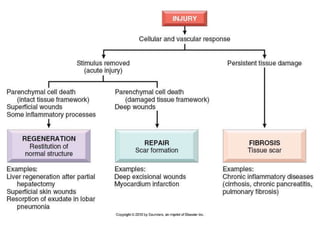
1) Tissue repair involves proliferation of cells from remnants of injured tissue, blood vessels, and fibroblasts to form new tissue.
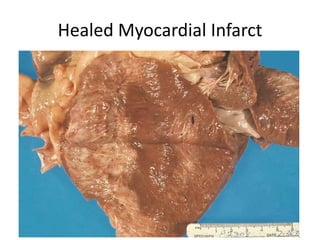
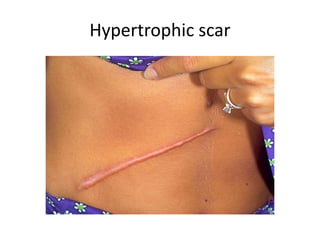
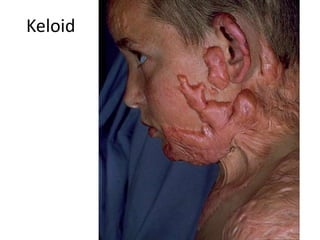
2) Repair can occur through regeneration of cells or through connective tissue replacement involving scar formation.
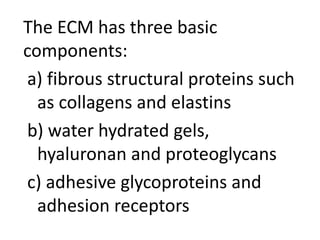
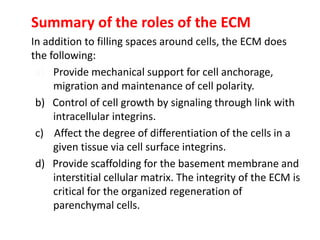
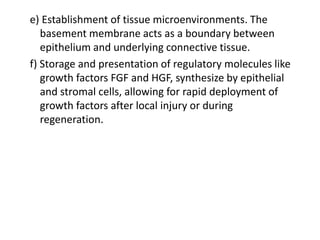
3) The extracellular matrix is essential for repair, providing structure and signaling molecules to support cell growth and tissue organization.