1. The document discusses evidence that principal energy levels contain sub-levels with different energies, as aluminum's ionization energy is lower than magnesium's despite both being in the third principal level.
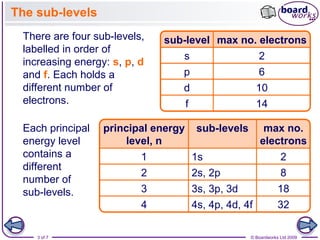
2. There are four sub-levels - s, p, d, and f - ordered by increasing energy, each holding a different maximum number of electrons.
3. Niels Bohr developed the Aufbau principle stating that electrons occupy sub-levels from lowest to highest energy, so 1s fills before 2s, 2p before 3s, 3p before 3d, and 4s before 3d.