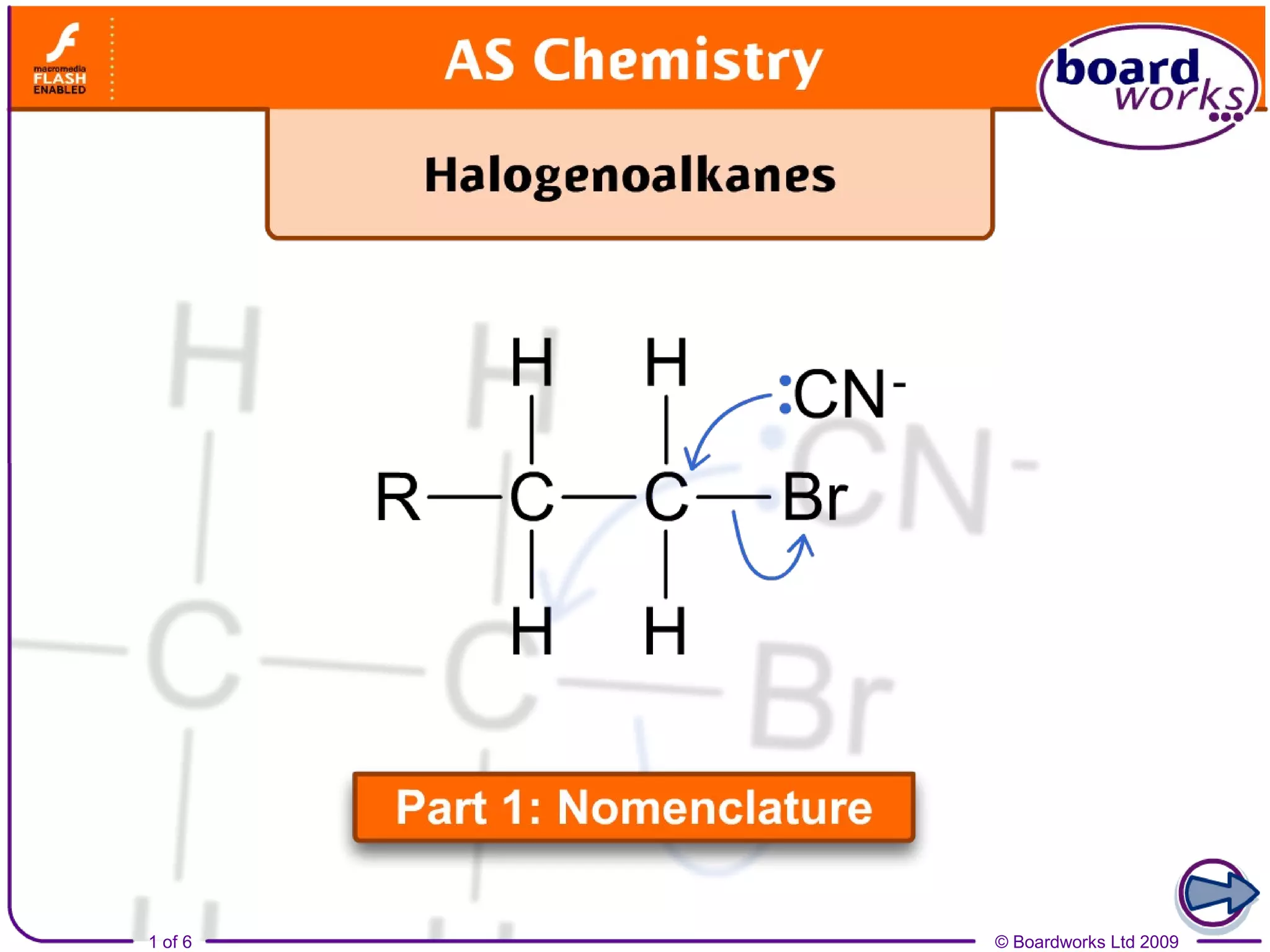
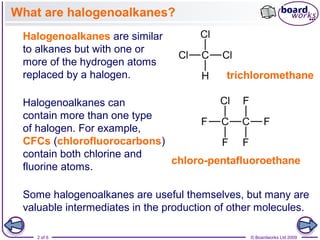
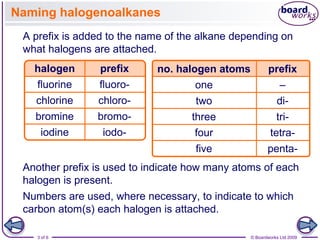
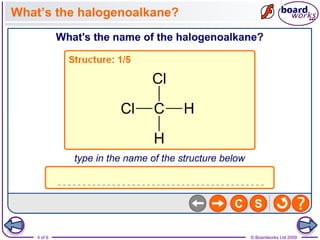
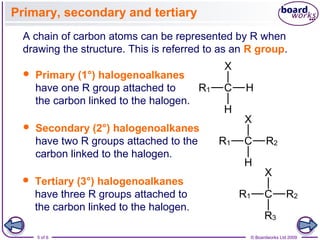
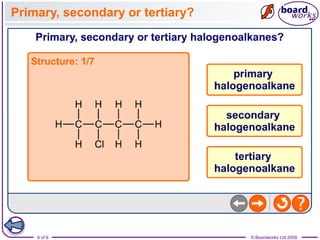
Halogenoalkanes are organic compounds where one or more hydrogen atoms in an alkane are replaced by halogen atoms such as fluorine, chlorine, bromine or iodine. Naming halogenoalkanes involves using prefixes to indicate the halogen atom and numerals to indicate the number and location of the halogen substitution. Halogenoalkanes can be classified as primary, secondary or tertiary depending on whether the carbon atom bonded to the halogen has one, two or three carbon substituents respectively.