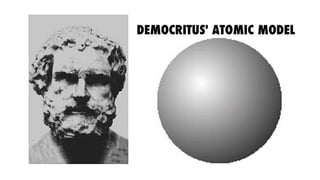
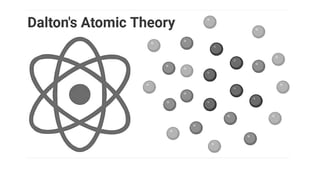
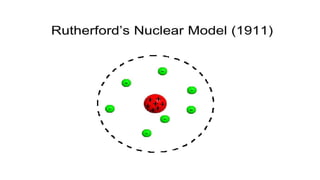
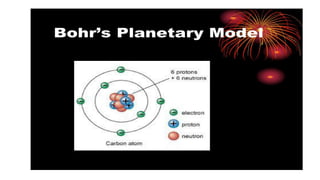
The document outlines the evolution of atomic models from ancient to modern times, highlighting key scientists and their contributions, including Democritus, Dalton, Thomson, Rutherford, Bohr, and Schrödinger. Each model reflects advancements in scientific understanding, addressing limitations of previous models and incorporating new experimental evidence, culminating in the quantum mechanical model. The importance of revising theories based on new evidence is emphasized throughout the history of atomic theory.