This document provides an overview of pharmacovigilance in India, including:
- The history of pharmacovigilance efforts in India from 1986 to the present.
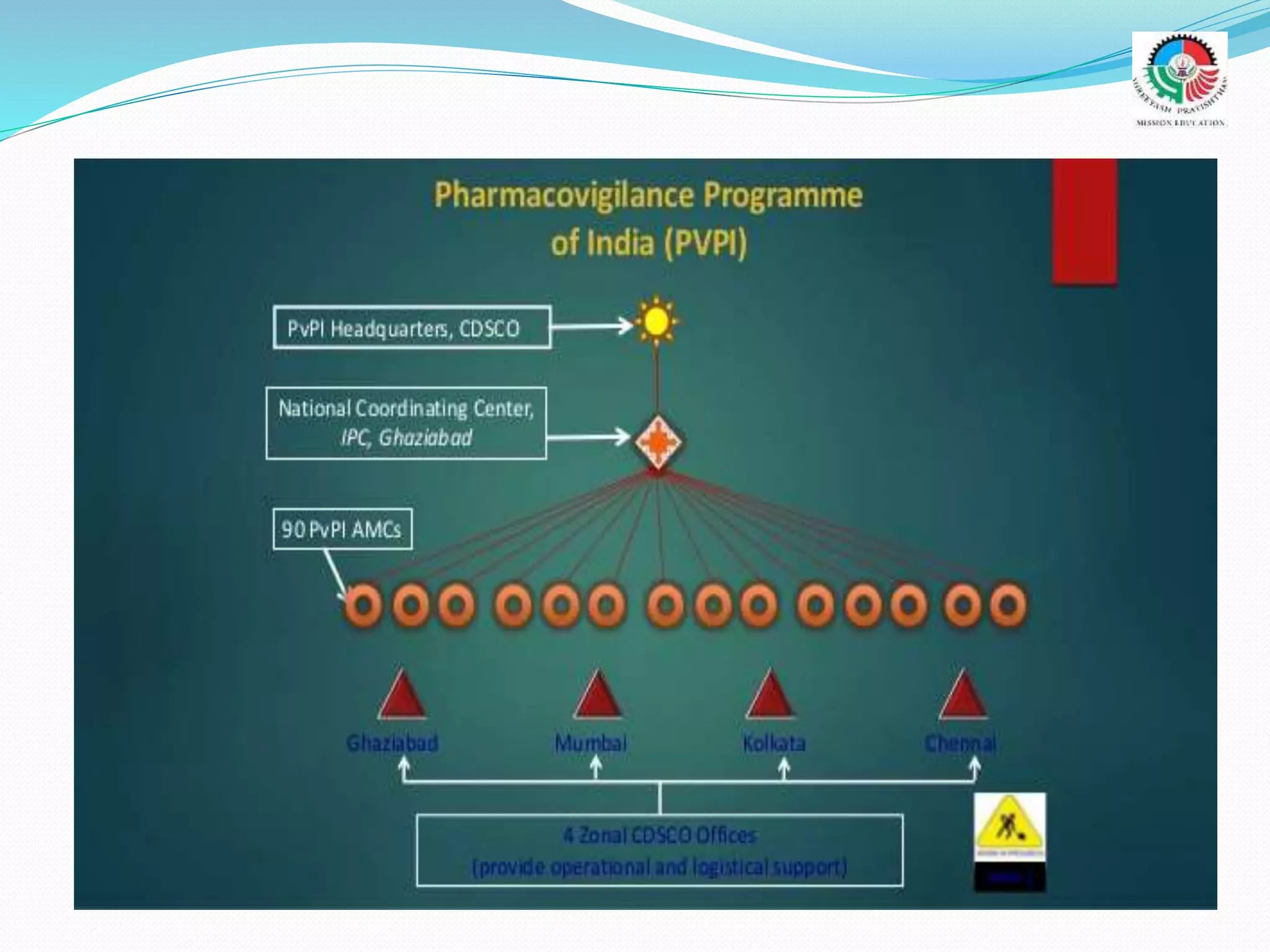
- The objectives and goals of the current Pharmacovigilance Program of India (PvPI), including establishing a nationwide safety reporting system and expanding electronic reporting.
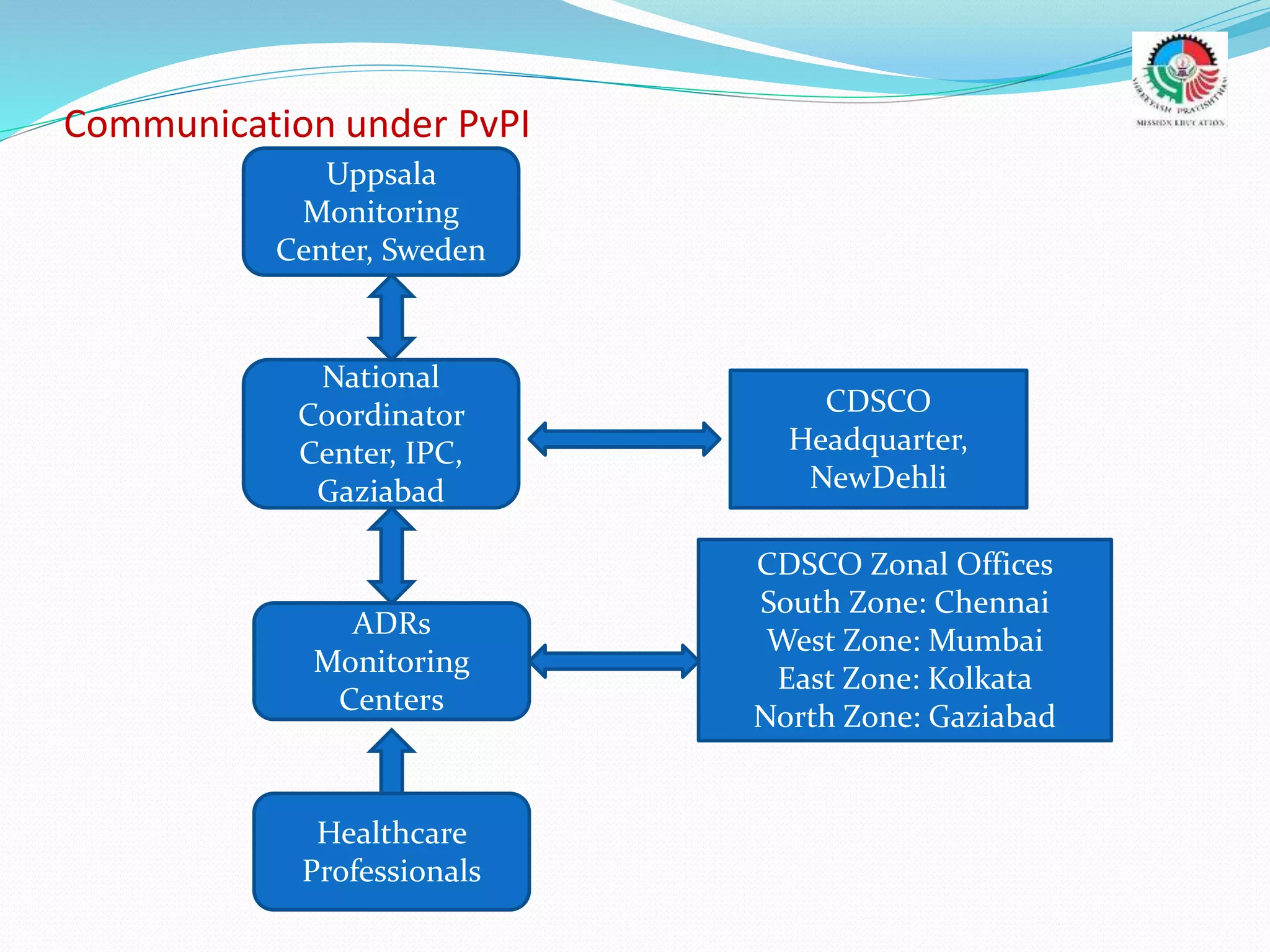
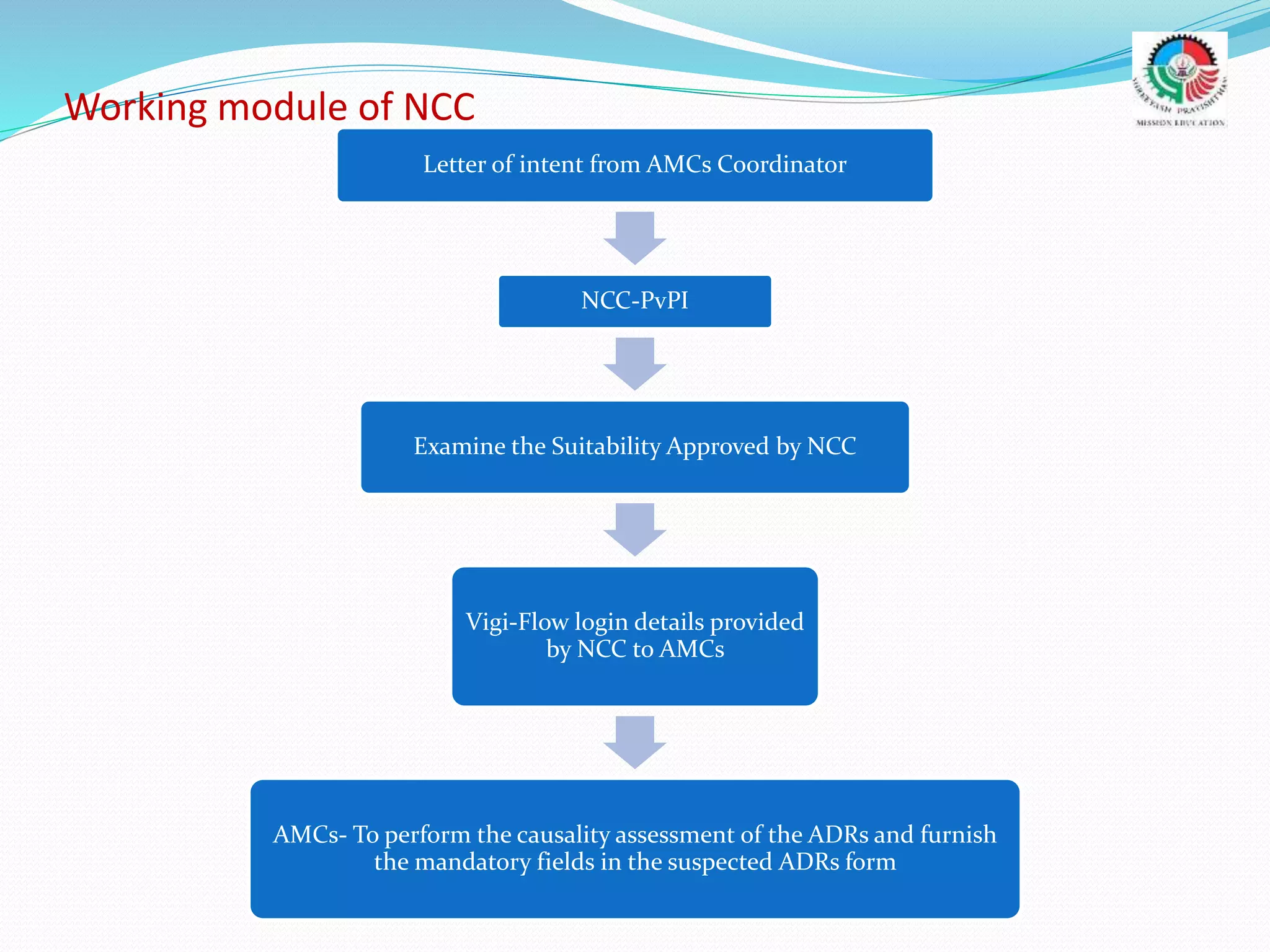
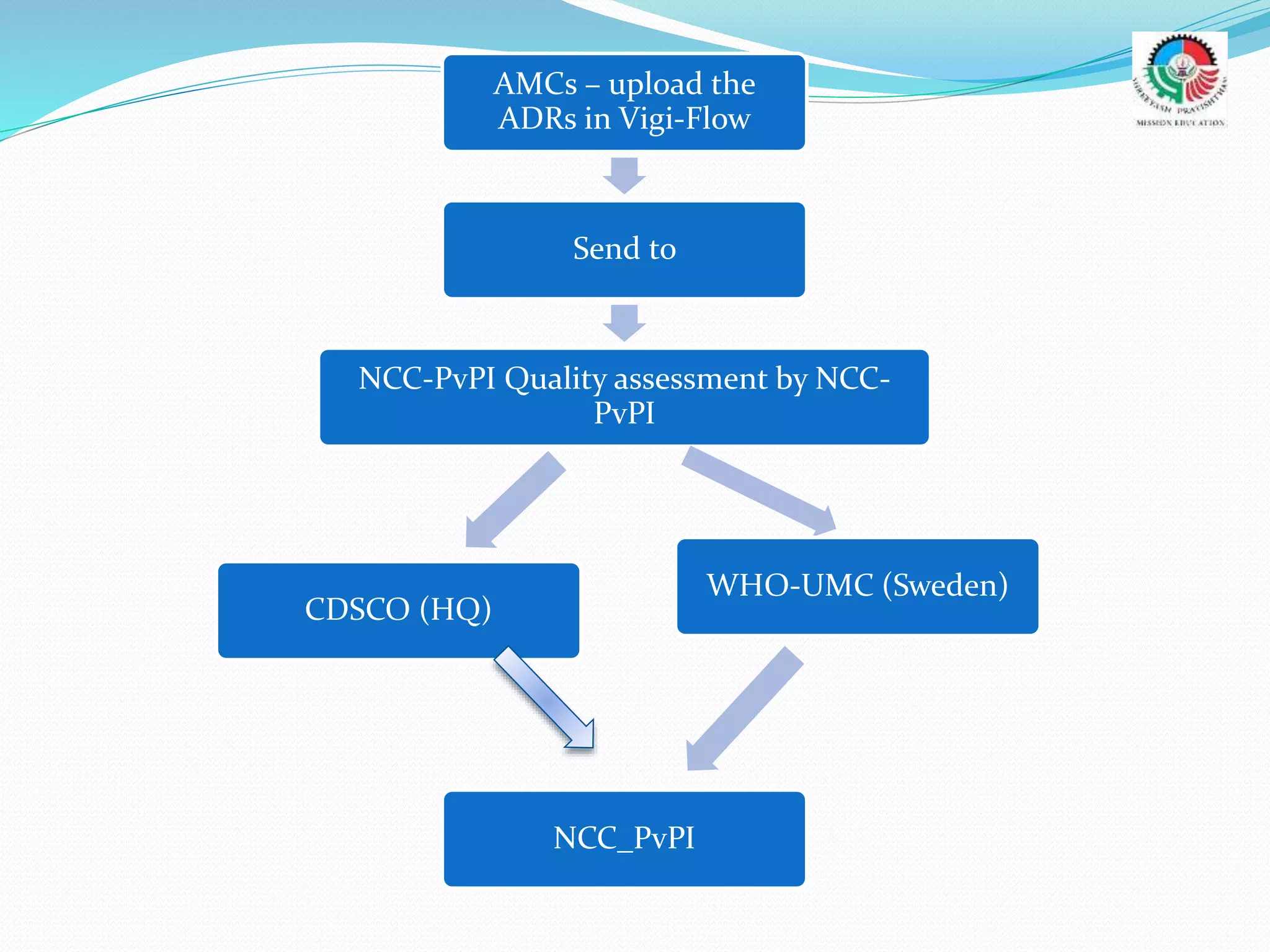
- The roles and responsibilities of stakeholders like the National Coordinating Center, Advisory Monitoring Centers, and CDSCO in the PvPI.