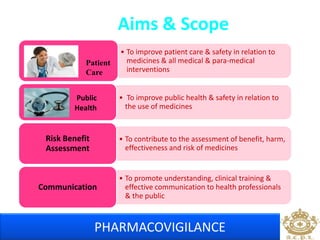
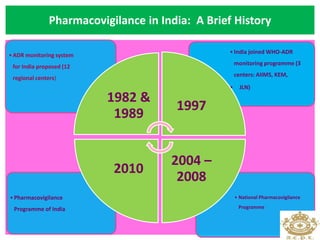
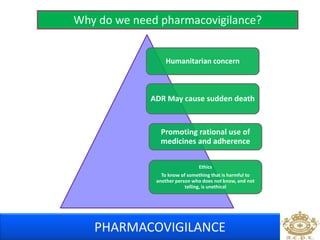
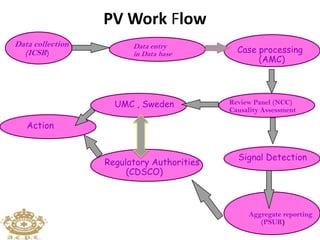
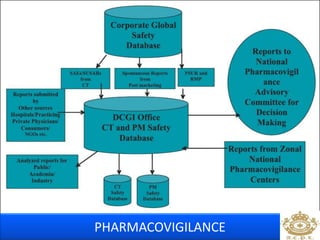
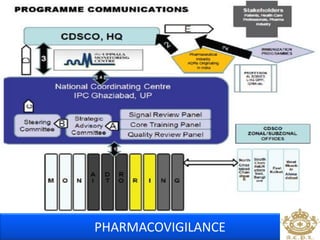
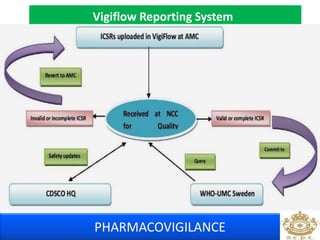
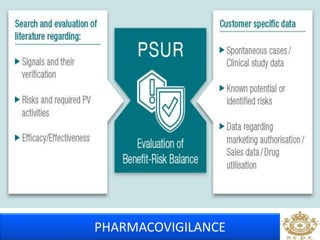
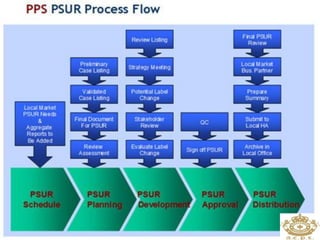
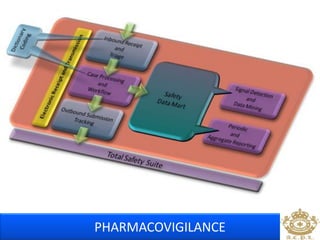
This document discusses pharmacovigilance, which is the science of monitoring the safety of pharmaceutical drugs. It aims to improve patient safety, public health, assess risk and benefits of drugs, and promote effective communication. A functional pharmacovigilance system requires a national center, reporting system, database, advisory committee, and communication strategy. Any adverse drug reactions or lack of efficacy should be reported. Periodic safety update reports provide an evaluation of the risk-benefit balance of drugs and are submitted to regulatory authorities on defined timelines.