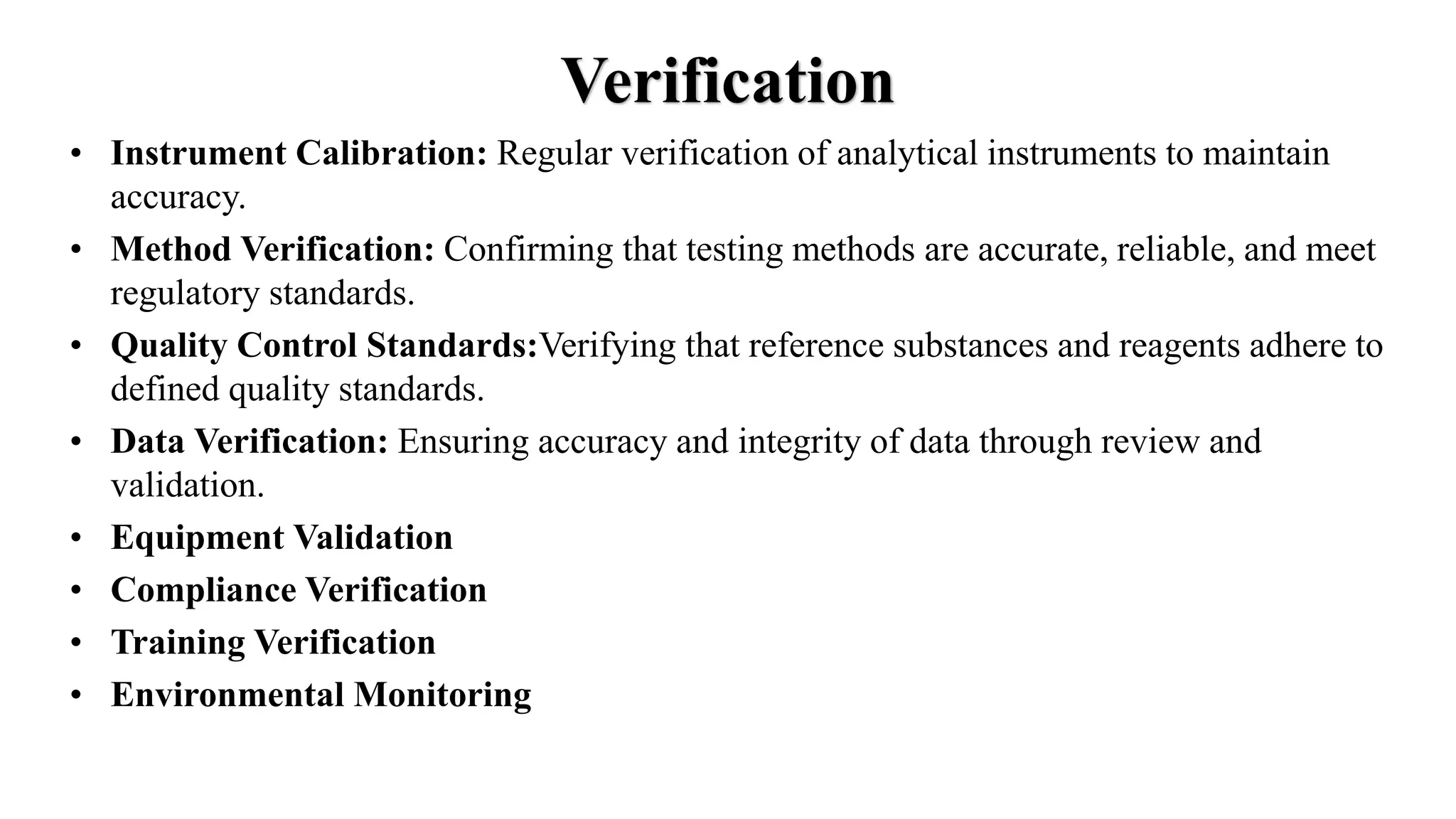
This document summarizes the functions of a pharmaceutical quality control laboratory. It discusses that quality control ensures manufactured products adhere to defined quality standards and are safe, effective and of good quality. The document then outlines various aspects of operating a quality control lab, including complying with good laboratory practices (GLP), good manufacturing practices (GMP) and ISO/IEC 17025. It discusses documentation, personnel, equipment, contracts, reference substances, calibration, verification, and qualifications. Finally, it summarizes procedures for sampling, testing, validating, evaluating and reporting analytical results.