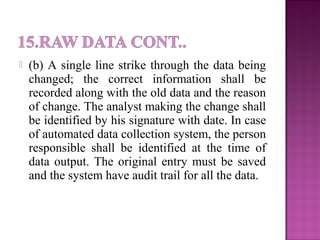
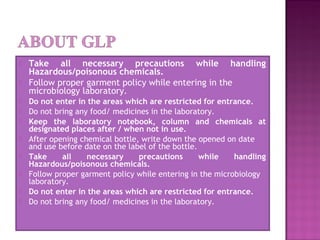
This document outlines Good Laboratory Practice (GLP) regulations which provide a framework for laboratory studies. It discusses general GLP requirements including laboratory premises, personnel, equipment, chemicals and reagents, housekeeping, maintenance, calibration, reference materials, microbiological cultures, quality systems, audits, standard operating procedures, protocols, raw data storage and archiving. Specific requirements are provided for laboratory design, equipment, chemicals, safety practices, calibration, reference standards, microbial cultures, quality systems, audits and corrective actions. Internal audits are conducted periodically to ensure ongoing compliance.













































































![ Flush the complete system (without column) with warm water
(40°) once in a week using union instead of column.
If the system suitability is not achieved by the column then
regenerate the column as per manufacturer’s instructions / SOP for
column generation.
Purge seal wash line, injector and all the inlets of pump with water
before use.
Sonicate all ports inlet filter with [water: methanol (50:50)] once in a
week.
Check the tubing/Syringe for air bubbles of all ports.
Check the valves for precipitation & clogging.
Clean all the inline filters, frits & check valve cartridges at least
twice in a month.
Before converting the system to normal phase wash with water/
Methanol/ IPA/ Diluent serially.
After completion of analysis, follow reverse procedure to
convert to reverse phase.](https://image.slidesharecdn.com/schedule-l1-170304110752/85/Schedule-L1-82-320.jpg)